Which Equation Represents A Neutralization Reaction 6 Hclo
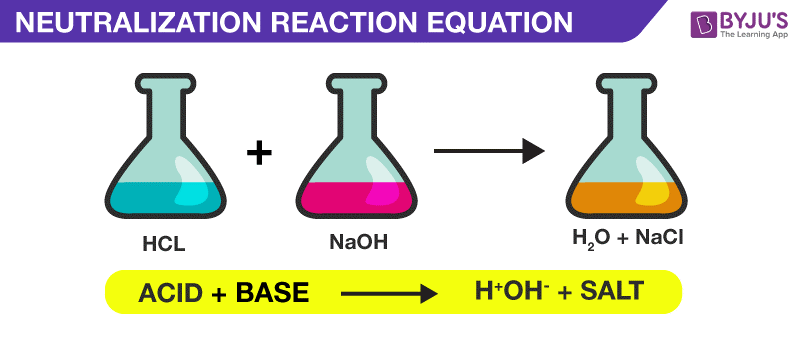
Hey there, science curious friends! Ever feel like the world of chemistry is a bit… well, complicated? I get it. Sometimes those fancy chemical names and equations can feel like they're speaking a secret language. But what if I told you that understanding a little bit of chemistry could actually make your everyday life more fun? Seriously! It’s not just about beakers and explosions (though, okay, that can be fun too). Today, we’re going to tackle something super cool and surprisingly relevant: neutralization reactions.
Now, before you start picturing litmus paper and grumpy professors, let’s get one thing straight. Neutralization reactions are all about finding that perfect balance. Think of it like a perfectly mixed smoothie, or a catchy song with just the right harmony. It’s when two things, that might seem a little too much on their own, come together to create something calm, collected, and perfectly… well, neutral!
So, what’s the big question we’re diving into today? It’s a fun one: Which equation represents a neutralization reaction? And then, we’re going to zero in on a very specific one: 6 HClO. Now, that might look like a bunch of random letters and numbers to you, but trust me, it holds a little secret about how things get balanced in the chemical world.
The Chemistry of Balance: What's a Neutralization Reaction Anyway?
Let’s break it down. Imagine you have something that’s a little too acidic. Think of that sharp, sour taste of a lemon, or the tingle you get from a fizzy drink. Acids are great for certain things, but too much of them can be a problem. On the flip side, you have things that are basic (or alkaline). These can feel a bit slippery, like soap, and are the opposite of acids. Too much of a base can also be… well, not so great.
A neutralization reaction is when you take an acid and mix it with a base. What happens? Abracadabra! They react, and the harshness of both is canceled out. They become neutral. It’s like they’ve had a little chat, understood each other, and found common ground. And usually, the end result is something pretty harmless, like water and a salt. Pretty neat, right?
Think about it in your kitchen. If you accidentally spill something acidic, like lemon juice, on your counter, and it feels a bit sticky or harsh, what might you use to clean it up? Often, you’d reach for something like baking soda. Baking soda is a base! When they meet, they fizz and bubble – that’s the reaction happening! – and they neutralize the acidity, making your counter clean and safe.
Decoding the Mystery of 6 HClO
Now, let’s get to our star of the show: 6 HClO. What does this little scientific snippet actually mean? Let’s unpack it. The HClO part is the chemical formula for a specific acid. It's called hypochlorous acid.

Hypochlorous acid sounds a bit intimidating, doesn’t it? But this is a compound you actually encounter more often than you might think! It’s actually the active ingredient in many common disinfectants and bleaches. Ever use a spray to clean your bathroom or kitchen surfaces to kill germs? Chances are, hypochlorous acid was involved in making sure those surfaces are sparkling and germ-free.
And the 6? Well, in chemistry, numbers in front of a chemical formula (like that 6) tell us how many molecules of that substance we have. So, 6 HClO simply means we have six molecules of hypochlorous acid. It’s like saying you have six individual lemons instead of just one.
Putting HClO to the Neutralization Test
So, how does our friend hypochlorous acid (HClO) fit into a neutralization reaction? Remember, for neutralization, we need an acid and a base. Hypochlorous acid, as its name suggests, is an acid. So, to neutralize it, we need to introduce a base.
A common and simple base we often use in these types of reactions is something like sodium hydroxide (NaOH). It’s a strong base, and it’s ready to tango with our acid. When HClO meets NaOH, something magical happens.
The hydrogen ion (H⁺) from the acid happily pairs up with the hydroxide ion (OH⁻) from the base. And what do you get when you combine H⁺ and OH⁻? You guessed it: water (H₂O)! Yay, water! It’s that essential stuff that keeps us all alive.

But wait, there’s more! The remaining parts of the acid and the base also find each other. In this case, the chlorine (Cl) and the sodium (Na) ions get together to form a salt. Specifically, they form sodium hypochlorite (NaClO). Now, this salt is different from the table salt (sodium chloride, NaCl) you sprinkle on your fries, but it's still a salt nonetheless! In this specific reaction, the salt formed (NaClO) is actually the main component of liquid bleach. So, even the stuff that cleans your sink is a product of a neutralization reaction!
The Equation Unveiled!
So, what’s the equation that shows this whole process? For a single molecule of hypochlorous acid reacting with a base like sodium hydroxide, it looks like this:
HClO + NaOH → NaClO + H₂O
This equation tells us that hypochlorous acid plus sodium hydroxide produces sodium hypochlorite and water. It’s a beautiful dance of atoms and molecules, resulting in a calmer, more balanced outcome.
Now, back to our original question: Which equation represents a neutralization reaction with 6 HClO? If we have six molecules of HClO, we’d also need enough base to neutralize all of them. So, if we stick with NaOH as our base, the balanced equation would be:
6 HClO + 6 NaOH → 6 NaClO + 6 H₂O
See? The numbers just scale up. We have six units of acid, six units of base, and they produce six units of salt and six units of water. It’s still the same fundamental reaction, just on a slightly larger scale. It's like baking six cookies instead of one – the recipe (the reaction) is the same, you just use more ingredients!
Why This Matters (and How It Makes Life More Fun!)
Okay, so we’ve talked about acids, bases, and how they cancel each other out. Why is this exciting? Because understanding these basic principles can demystify so many things around you!
Think about it. That disinfectant you use? Neutralization. The antacids you take when your stomach is feeling a bit too acidic? Neutralization! Even the way our own bodies work, maintaining a precise pH balance, relies on these fundamental chemical principles.

It’s also about problem-solving and efficiency. When you understand that acids and bases neutralize each other, you can start to think about practical applications. Need to adjust the pH of a swimming pool? Neutralization. Want to clean something without using harsh chemicals? Understanding neutralization can guide you to safer alternatives.
It’s like learning a new superpower. Suddenly, those everyday cleaning products, those fizzy drinks, even the natural world around you, starts to make a little more sense. It’s not just magic; it’s chemistry, and it’s happening all the time!
And honestly, knowing these things can make you feel a little bit more empowered. You can look at a label, understand a little more about what’s in it, and make more informed choices. It’s a tiny step towards understanding the incredible complexity and beauty of the world we live in.
The Journey Continues…
So, there you have it! The equation representing the neutralization of hypochlorous acid involves an acid meeting a base, and the result is a delightful mix of salt and water. It’s a chemical hug, if you will, where opposing forces come together to create harmony.
This is just the tip of the iceberg, my friends. The world of chemistry is vast and full of wonders, and understanding even these small concepts can unlock a whole new way of looking at things. So, don’t be afraid to keep asking questions, to keep exploring, and to keep learning. The more you learn, the more fascinating your world becomes. Who knows what other amazing chemical adventures await you?
