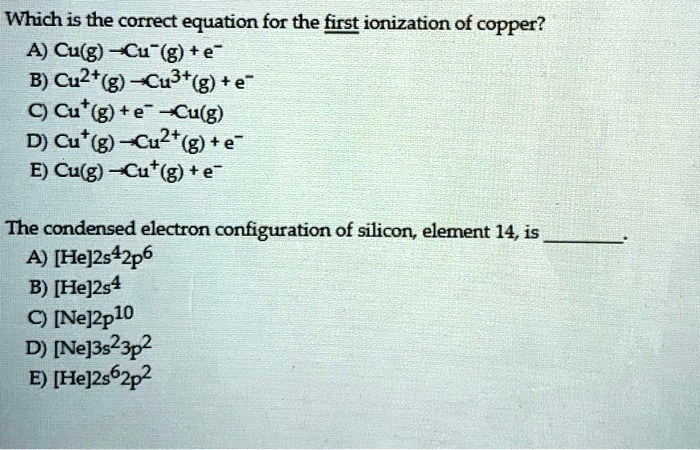
Which Equation Correctly Represents The First Ionization Of Copper
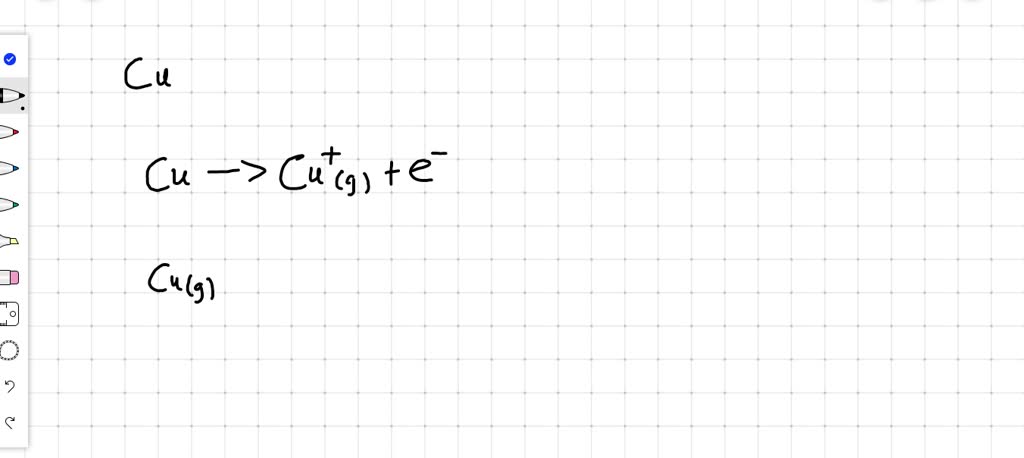
Ever wondered about the tiny, invisible world that makes up everything around us? From the shiny coins in your pocket to the vibrant colors in a sunset, it's all thanks to the fascinating behavior of atoms. And when it comes to atoms, there's a particularly interesting element called copper. Understanding how copper behaves, especially when it comes to losing an electron, is like unlocking a little secret of chemistry. It's a fun peek into the building blocks of our universe, and surprisingly useful too!
So, what exactly is the first ionization of copper? Think of an atom like a tiny solar system, with a nucleus at the center and electrons orbiting around it. Ionization is simply the process of an atom losing an electron. The "first ionization" just means it's losing its very first electron. Why is this cool? For beginners, it's a stepping stone into understanding chemical reactions. For families, it’s a way to spark curiosity in kids about science by talking about how elements behave. Hobbyists, especially those interested in electronics or materials science, might find this knowledge helps them understand why copper is such a great conductor of electricity – it’s because it readily gives up an electron!
Now, let's get to the exciting part: the equation! In chemistry, we use equations to show what's happening. For the first ionization of copper, we're showing a neutral copper atom (represented by the symbol Cu) losing one electron to become a positively charged copper ion (Cu⁺). We also show the electron being released (represented by e⁻). So, the equation looks like this:
Cu (g) + Energy → Cu⁺ (g) + e⁻
What does this mean? The (g) after Cu and Cu⁺ stands for 'gas'. This is because ionization is often studied when the element is in a gaseous state. The Energy part is crucial. It takes a certain amount of energy to pull that electron away from the atom. This energy is called the first ionization energy.
You might also see variations, like discussions about the second ionization energy of copper, where the atom loses a second electron (becoming Cu²⁺). This takes even more energy because the remaining electrons are held more tightly by the nucleus. Or, you might see this process discussed in the context of electrolysis, where electrical energy is used to force atoms to lose or gain electrons.
Getting started with this concept is easier than you think! You don't need a fancy lab. Start by looking up ionization energies for different elements online. You'll see a table of numbers and can compare how easily different elements lose electrons. Try explaining it to a friend or family member – teaching is a great way to learn! You can even find fun videos online that use animations to show atoms losing electrons.
Understanding the first ionization of copper is a small window into the vast and dynamic world of chemistry. It’s a simple equation that reveals fundamental properties of an element we encounter every day. It's a testament to how even the smallest interactions at the atomic level have big implications. So, the next time you see a copper wire or a shiny penny, you can appreciate the little chemical dance happening within!
