Which Energy Graph Represents The Nonspontaneous Transition

Hey there, fellow curious minds! Ever stare at a bunch of graphs and wonder, "What's the deal with this one?" Today, we're diving into a little mystery, a puzzle of energy and change, all about those tricky nonspontaneous transitions. Sounds a bit sci-fi, right? But stick with me, it's actually super relatable, and honestly, pretty cool when you wrap your head around it.
So, what are we even talking about? Imagine you've got a ball at the top of a hill. It's just begging to roll down, right? That's a spontaneous process. It happens all on its own, no pushing required. Think of a melting ice cube on a warm day, or sugar dissolving in your coffee. Easy peasy.
But then there are the other guys, the nonspontaneous ones. These are the transitions that are like trying to push that ball uphill. They don't want to happen. They require a little nudge, a bit of an energy boost, to get going. Think about charging your phone – it doesn't just magically fill up with power, does it? You have to plug it in and supply it with electricity. That's a nonspontaneous process in action!
Now, imagine we're looking at a graph that's trying to tell us the story of these energy changes. It’s like a little map of what’s happening behind the scenes. And we’re on a quest to find the graph that best represents one of these nonspontaneous transitions.
What Are We Looking For in This Energy Graph Mystery?
When we talk about energy in these kinds of processes, we often think about something called Gibbs Free Energy. Don't let the fancy name scare you! Think of it as the "usable" energy that’s available to do work. It's like the pocket money you have to spend on fun activities.
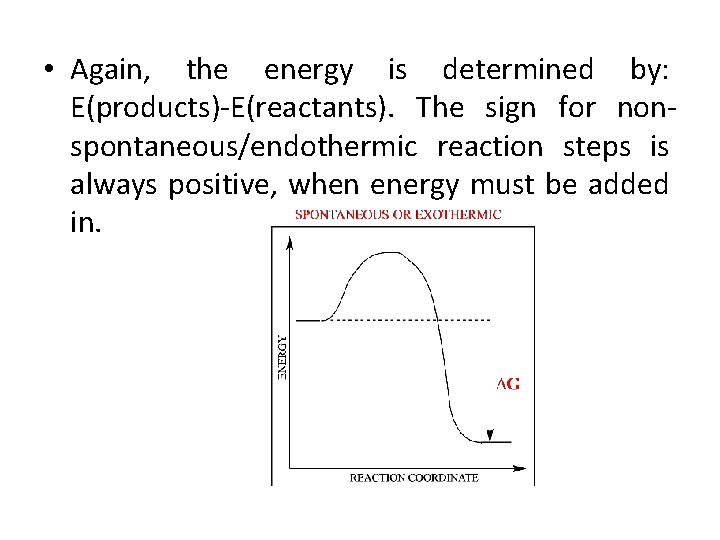
For a process to happen on its own (spontaneously), the Gibbs Free Energy needs to decrease. It’s like your pocket money naturally going down as you buy that awesome ice cream. For a nonspontaneous process, the opposite has to happen. The Gibbs Free Energy has to increase. You have to put more money in your pocket to buy that ice cream than you had to begin with. Weird, right? But that’s how the universe keeps us on our toes!
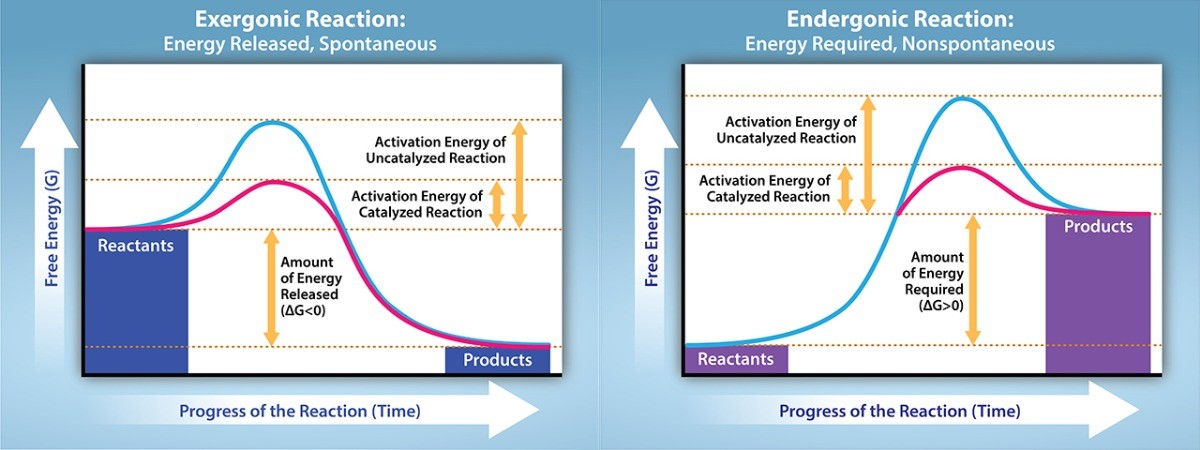
So, our mission, should we choose to accept it, is to find a graph that shows this increase in Gibbs Free Energy from the starting point (the reactants) to the ending point (the products).
Let’s Play "Spot the Graph"!
Imagine we have a few different graphs. They might show energy levels on the y-axis and the "progress of the reaction" or "reaction coordinate" on the x-axis. This x-axis is just like a timeline of the reaction happening.
One graph might show the energy going down, like a gentle slope. That’s a spontaneous process, and it definitely isn't our target. No thanks, we’re looking for something that fights against the natural flow!
Another graph might show the energy staying pretty much the same. That’s like a perfectly balanced seesaw – not much is happening, or it’s in a state of equilibrium. Interesting, but not quite our nonspontaneous hero.
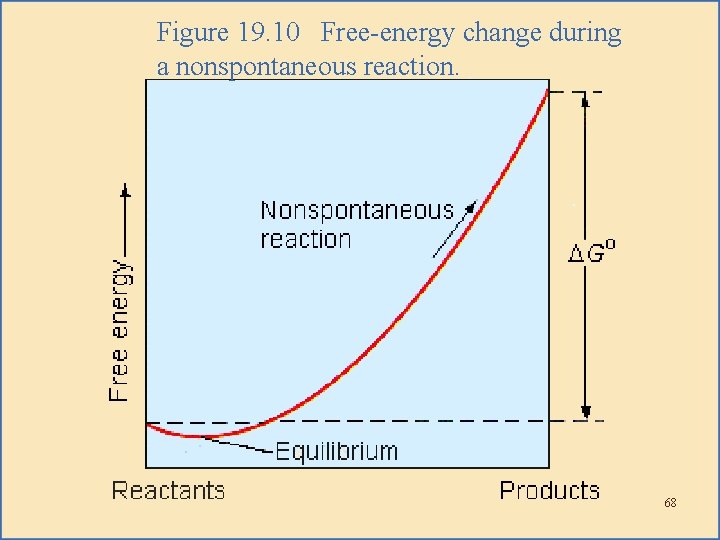
Then, there’s the one we’re looking for! This graph will show the energy level at the start, and then, as the reaction progresses, the energy level will climb uphill. It’s like looking at a graph of your bank account after you’ve spent way too much on online shopping – it goes down! But if you’re trying to save money, you have to actively put more in to increase that balance. That’s the spirit we’re after!
So, we're looking for a graph where the final energy state is higher than the initial energy state. The line on the graph will be trending upwards from left to right, representing an energy input is required. It’s like the universe is saying, "Nope, this isn’t going to happen by itself. You gotta give me something here!"
Why is This Even a Thing? (And Why Is It Cool?)
You might be thinking, "Why bother with things that don't want to happen naturally?" Well, that's where things get really interesting! Many of the most important processes in life and technology are nonspontaneous. Our bodies performing complex tasks, plants growing from tiny seeds, batteries powering our devices – these are all powered by overcoming energy barriers.
Think about photosynthesis. Plants take simple things like sunlight, water, and carbon dioxide and create sugars. This process is definitely not spontaneous! It requires the energy from sunlight to drive it. Without that external energy, plants wouldn't be able to grow and thrive.
Or consider building a sandcastle. The sand is all spread out on the beach, right? To build a nice, structured sandcastle, you have to actively gather the sand, add water, and shape it. That’s work! You’re increasing the order and energy of the system, fighting against the natural tendency of the sand to just be a messy pile.

These nonspontaneous transitions, when represented by an upward-sloping energy graph, are like the building blocks of progress. They show us that with a little effort, a bit of added energy, we can achieve amazing things that wouldn't happen on their own.
It's like the difference between letting your room get messy (spontaneous!) and cleaning it up and organizing it (nonspontaneous, but way more satisfying!). The graph for cleaning your room would show an increase in "tidiness energy" or a decrease in "mess energy" – essentially, you're investing effort to achieve a more ordered state.
So, when you see a graph where the energy levels rise from the start to the finish, don't think of it as a failure. Think of it as an opportunity. It’s the universe saying, "This requires a bit of a boost, but the outcome can be pretty incredible!" It's the story of innovation, of life, of building something new.
Ultimately, identifying the graph that represents a nonspontaneous transition is about understanding that not everything in the universe is a free ride. Some of the most impactful changes require us to actively participate, to provide that spark, that extra energy. And that, my friends, is a pretty neat concept to ponder.
