Which Element Has The Greatest Number Of Valence Electrons Apex

Hey there, science adventurers! Ever wonder about the tiny, invisible parts that make up everything around us? We're talking about atoms, the building blocks of the universe. And within these atoms, there are these super important things called valence electrons. Think of them like the VIP guests at an atom's party – they're on the very outside, and they're the ones who get to mingle and make connections.
So, the big question that might be tickling your brain is: which element is the ultimate champion when it comes to these party-hopping valence electrons? It's like asking who has the most friends at the school dance! And guess what? The answer is both super simple and incredibly cool.
Before we reveal the champ, let's quickly chat about why valence electrons are such a big deal. They're not just chilling out; they're the secret sauce behind how atoms stick together to form molecules. Everything you see, touch, and even breathe is thanks to these energetic little guys.
Imagine atoms as tiny solar systems. The nucleus is the sun, and the electrons orbit around it like planets. Valence electrons are the planets on the very outermost orbit. They're the ones that can easily escape or join up with other atoms.
Now, for the grand reveal! The element that proudly boasts the greatest number of valence electrons is none other than noble gases. But wait, it's not just one element! It's a whole group of elements that share this special superpower.
The noble gases are a collection of elements found in the last column of the periodic table. They're famous for being a bit… aloof. They don't usually like to interact much with other elements. And there's a really good reason for that!
These elements, like Helium, Neon, Argon, Krypton, Xenon, and Radon, are all incredibly stable. They're like the cool kids who already have all the friends they need. They don't need to go out and make new connections because their outer shells are already perfectly full.
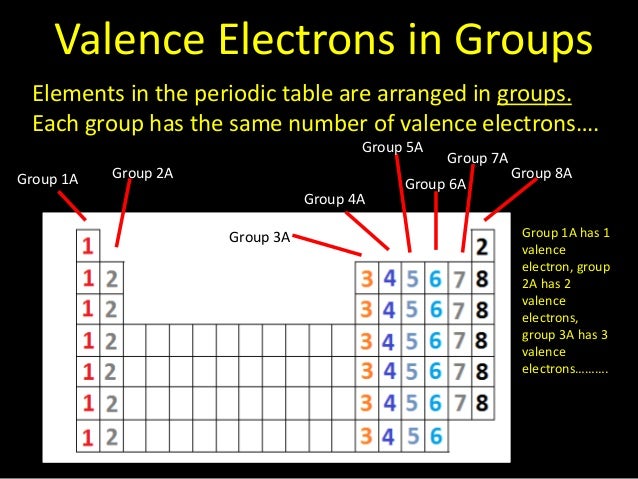
So, how many valence electrons do they have? Most of them have a whopping eight valence electrons! That's like having a full house of friends at your party – no room for anyone else, and perfectly content as is. This "full house" is known as a stable octet, and it’s the dream of many an atom.
Why is eight so special? Well, in the world of atoms, having eight valence electrons is like reaching the ultimate level of satisfaction and security. They're so happy with their electron count that they rarely bother to share, steal, or form bonds with other atoms. They’re perfectly balanced!
Think about it: if you have the best toy ever, you probably don't feel the need to ask your friends to borrow theirs, right? Noble gases are kind of like that. They've got their perfect set of valence electrons, and they're perfectly happy.
Now, there's a tiny exception to the "eight" rule, and that's Helium. Helium is a bit of a special case. It's a noble gas, but its outermost shell can only hold a maximum of two electrons. And guess what? Helium is perfectly content with its two valence electrons. It’s like a mini-noble gas, just as stable and happy!
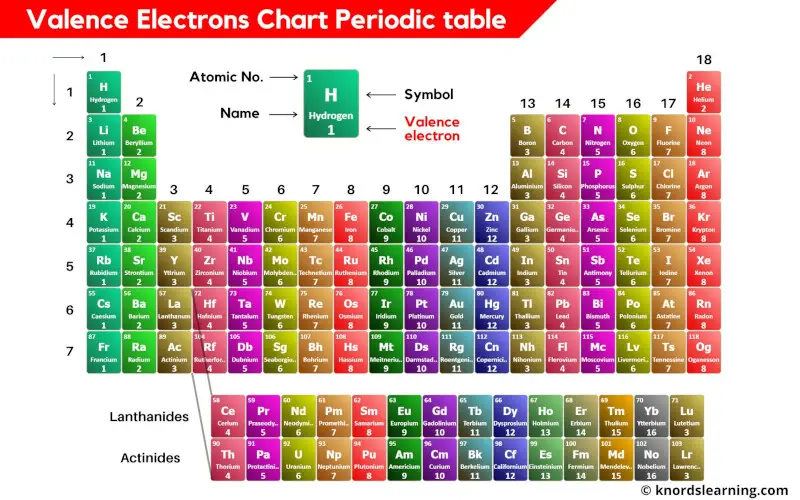
So, while most noble gases aim for eight, Helium is the sweet spot with just two. Both are considered full and therefore incredibly stable.
The fact that these elements have so many valence electrons (or a "full" shell, in Helium's case) makes them incredibly unreactive. This unreactivity is what makes them so special and useful in various applications. It's like having a super reliable, low-maintenance friend.
For instance, Neon, with its full outer shell of electrons, is used in those iconic glowing signs. It doesn't react with anything, so it just glows beautifully when electricity passes through it. Imagine a party where the decorations just light up on their own and don't get messy!
Argon is another noble gas that's incredibly useful. It's often used in welding to protect the metal from reacting with the air. It’s like a protective bubble that keeps things from getting compromised. Super handy, right?
And then there's Xenon. This gas is so stable and has such a distinctive glow that it's used in high-intensity headlights for cars and in some specialized lighting applications. It’s like having the brightest, most efficient flashlight that never gets hot!

The existence of these noble gases with their "complete" valence electron shells really helps scientists understand how other elements behave. By knowing that noble gases are so stable, we can better predict how elements with fewer valence electrons will try to gain, lose, or share them to achieve that same stable state.
It's like having a perfect score on a test. Everyone else wants to try and reach that score too, so they try different strategies to get there. The noble gases are the benchmark of electron happiness!
So, the next time you see a glowing neon sign or learn about how different elements form connections, remember the noble gases. They might seem a bit shy and standoffish in the world of chemistry, but their abundance of (or complete set of) valence electrons makes them the undisputed champions of stability and a really cool bunch of elements.
It’s their full outer shells that give them this unique personality. They’ve achieved electron harmony, and the universe benefits from their stable presence. Isn't it fascinating how something so small can have such a huge impact on everything around us?
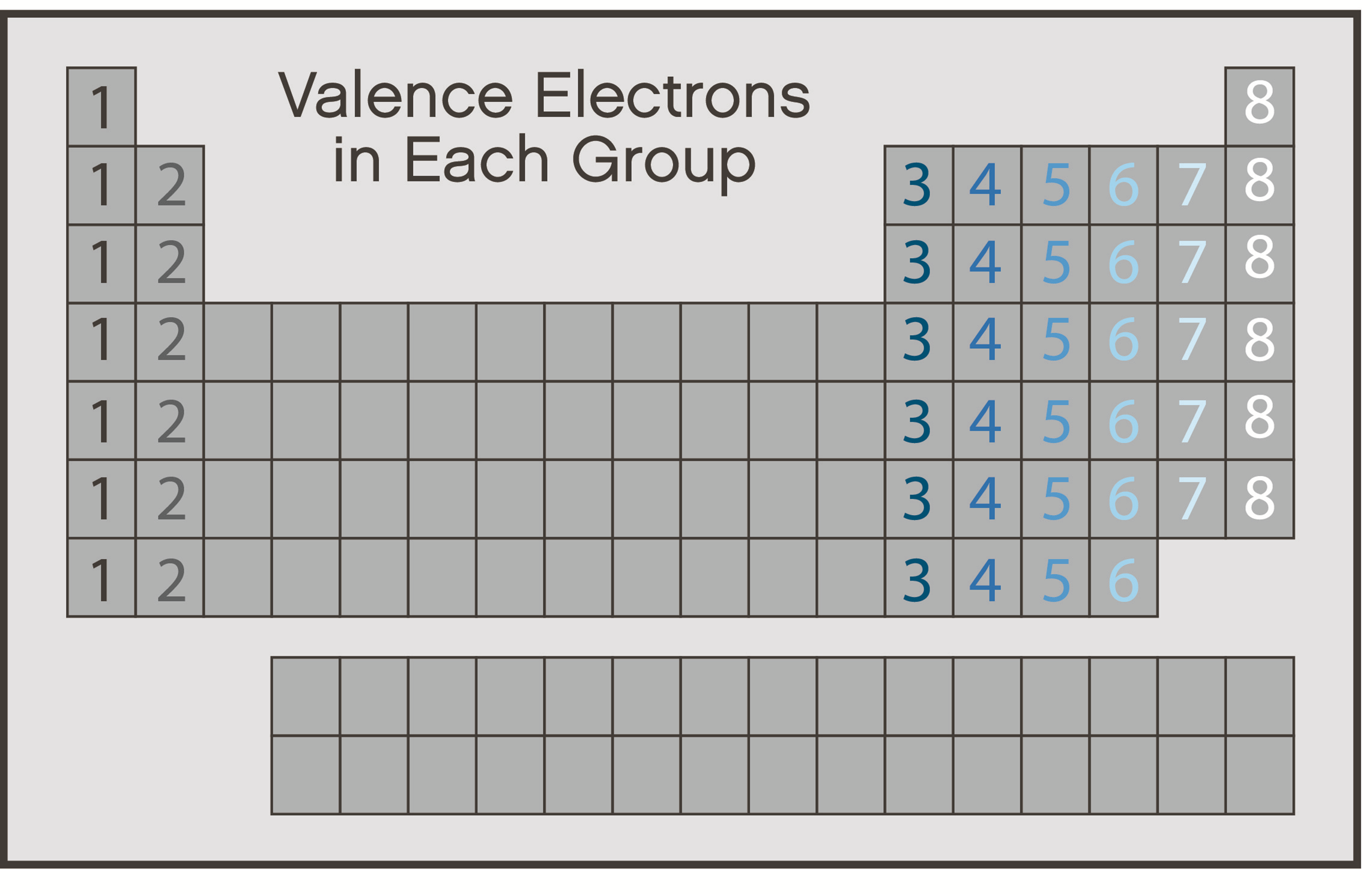
So, who has the greatest number of valence electrons? It's the whole gang of noble gases! They’re the VIPs of the electron world, with their full outer shells making them stable, unreactive, and incredibly fascinating. Keep an eye out for these special elements; they’re making a quiet but powerful impact everywhere!
Isn't chemistry just full of surprises? And the story of valence electrons and the noble gases is definitely one of the most delightful chapters. It’s a testament to how even the most seemingly unremarkable elements can have extraordinary properties.
So, there you have it! The element group with the greatest number of valence electrons, leading to ultimate stability and a cool, unreactive vibe, are the magnificent noble gases. Pretty neat, huh?
They're like the superheroes of the periodic table, not because they're flashy and reactive, but because they've achieved a perfect state of balance. And that, in itself, is pretty darn impressive.
