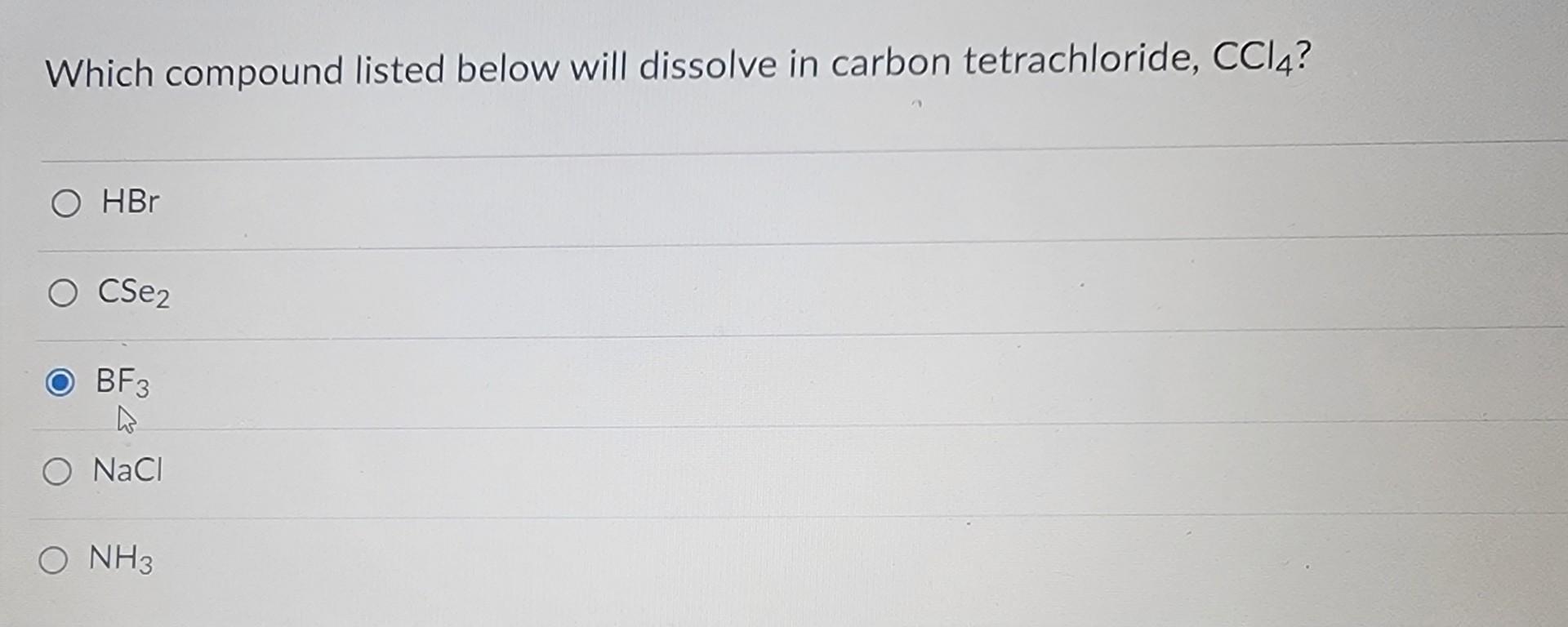
Which Compound Listed Below Will Dissolve In Carbon Tetrachloride Ccl4
Hey there, fellow curiosity seekers! Ever found yourself staring at a bunch of chemical names and wondering, "What's the scoop here? What's going to play nice with what?" Well, get ready for a little adventure into the world of dissolving, because we're diving into a question that's surprisingly fun: which of our listed friends will happily melt away in carbon tetrachloride (CCl4)?
Now, you might be thinking, "Dissolving? Sounds kinda… dry." But trust me, it's like a little chemistry party, and we get to be the VIP guests! Think of it like this: imagine you have a bunch of guests arriving at a party. Some people just naturally click, right? They love the same music, they're into the same kinds of snacks, and they just vibe. In chemistry, that same idea applies to which substances will mix and mingle. Some are natural besties, while others are like oil and water – they just don't blend.
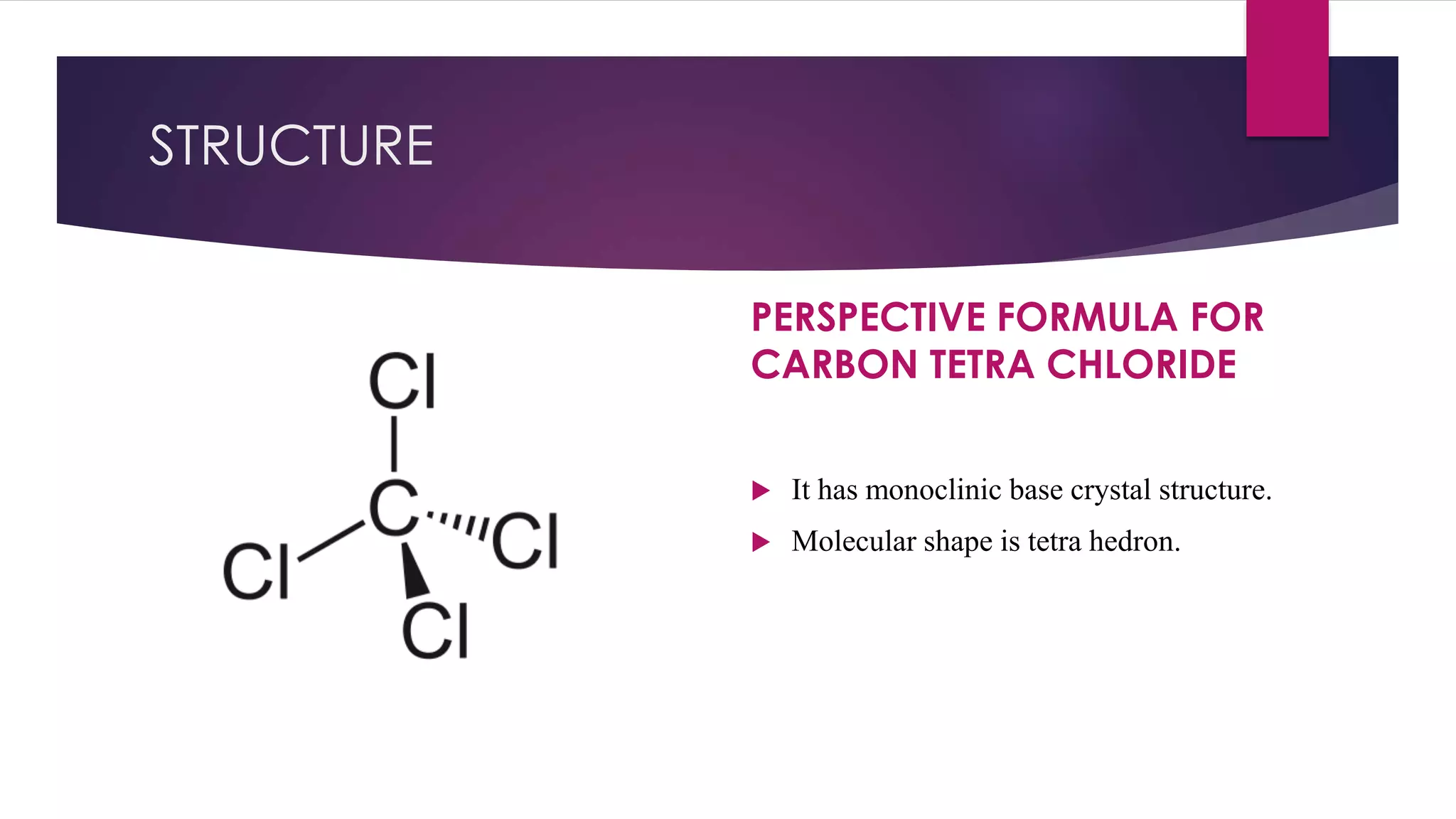
Our star of the show today is carbon tetrachloride (CCl4). It's this cool, colorless liquid. You might not have it lying around your kitchen, and that's a good thing! It's a bit of a powerhouse solvent. Think of it as the ultimate party host for certain types of molecules. It’s got this special personality that makes it really good at coaxing some things into dissolving. It's got a certain je ne sais quoi that other liquids just don't possess.
So, who are the lucky invitees to this CCl4 bash? We've got a lineup, and it's our mission to figure out who's going to be the life of the party. It's all about understanding who fits the vibe of our host, carbon tetrachloride. This isn't just about memorizing facts; it's about getting a feel for how these tiny particles interact. It’s like figuring out the secret handshake of the chemical world!
Let's break down the "why" without getting bogged down in super-sciencey jargon. You see, molecules have different personalities. Some are like magnets that are attracted to other magnets. Others are more… well, neutral. They don't have a strong positive or negative charge. Carbon tetrachloride (CCl4) has a rather neutral, or non-polar, personality. It’s like a laid-back host who’s happy to chat with anyone, but especially those who are also pretty chill and don't have any strong, opposing opinions.
This means that substances that are also non-polar are the ones most likely to dissolve in CCl4. They’re like kindred spirits! They get along famously. Imagine trying to mix oil with water. They just bounce off each other, right? That’s because oil is non-polar, and water is polar (it has those charged ends, like little magnets). But if you try to dissolve something like grease or wax in a suitable solvent, you'll see them disappear. That’s because they share that non-polar characteristic with the solvent.
Now, let's think about the contenders! We’re not going to list them all out here because the fun is in the discovery, right? But imagine a list that includes things like maybe some salts, some sugars, maybe some fats, or even some special plastics. It’s a real mix! And each one has its own unique molecular structure, its own way of being.
So, our mission, should we choose to accept it (and we totally should, because it’s fun!), is to pick out the one that shares that non-polar essence with carbon tetrachloride. It’s like a puzzle, but instead of fitting shapes, we're fitting molecular structures. We’re looking for the one that’s going to say, "Hey CCl4, you seem pretty cool. Let’s hang out!"
This whole dissolving business is pretty amazing when you think about it. It’s happening all around us, all the time! It’s how we make our morning coffee, how medicines get into our bodies, and how so many industrial processes work. And understanding it, even on a basic level, gives you a little peek behind the curtain of the universe.
When we look at our list of compounds, we're essentially scanning for that specific molecular "vibe." Some compounds are like over-enthusiastic party guests who are too charged up, too polar. They’re going to clash with the laid-back atmosphere of CCl4. Others are just not the right kind of molecule to even engage. They're perfectly happy in their own little worlds.

But then, there’s that one special compound. The one that's got that same mellow, non-polar signature. It's like finding your perfect dance partner on the dance floor. They just get each other. When this compound meets carbon tetrachloride, it’s a match made in molecular heaven. They embrace, they mix, they become one happy solution! You won't see them fighting or separating. Instead, they'll be happily dispersed, creating a beautiful, homogeneous mixture.
It’s this kind of insight that makes chemistry so utterly fascinating. It's not just about reactions and formulas; it’s about the relationships between these incredibly tiny building blocks of everything. And when it comes to carbon tetrachloride, it's a master at befriending other non-polar molecules. So, the next time you encounter a list like this, remember the party analogy. Who's going to feel most at home at the CCl4 chill-out zone?
Finding the answer is like uncovering a little secret. It’s a satisfying "aha!" moment when you pinpoint the compound that will dissolve. It shows you understand the fundamental principle: "like dissolves like." And in this case, our "like" is non-polar. So, go ahead, play detective with your chemical list. See if you can spot the one that’s going to be the perfect plus-one for carbon tetrachloride. It’s a small piece of chemical magic, and it’s incredibly rewarding to figure out!
The thrill of figuring out which compound will melt into our friend carbon tetrachloride (CCl4) is all about understanding molecular personalities. It's a delightful puzzle where "like dissolves like" reigns supreme, and our non-polar pals are the ultimate party-goers!
So, keep your eyes peeled for that special, non-polar compound. It’s out there, waiting to blend seamlessly with CCl4. Happy dissolving, and even happier discovering!
