Which Compound Has The Shortest Carbon-carbon Bond Length

You know how sometimes you're just, like, trying to get your thoughts together, and you need a moment of clarity? Well, in the wacky world of chemistry, molecules are doing the same thing, but instead of a deep breath, they're fiddling with their bond lengths. Specifically, we're talking about the tiny, invisible connections between carbon atoms – the building blocks of, well, pretty much everything organic, from your favorite pizza crust to your own DNA. Today, we're going on a little adventure to discover which carbon-carbon bond is the absolute shortest. Think of it as a molecular sprint to the finish line!
Now, before your brain starts picturing tiny little race cars zooming around, let's pump the brakes. Bond lengths aren't something you can see with the naked eye, or even a regular microscope. These are measured in angstroms, which are so tiny they make a grain of sand look like a giant boulder. We're talking about distances so small, they make the gap between your couch cushions seem like the Grand Canyon.
So, what exactly is a bond length? Imagine two friends, holding hands. The distance between their clasped hands is like a bond length. Now, imagine those friends are made of atoms, and their hands are their electron clouds, kind of zapping together. The stronger they grip, the closer they can get. And in the world of carbon-carbon bonds, there are a few different ways these atoms can "hold hands."
You've got your single bonds. Think of this as a relaxed, easygoing handshake. Two carbon atoms are happily linked, sharing a pair of electrons. It's like that friendly wave you give your neighbor when you're taking out the trash. Very casual, very standard. This is your everyday carbon-carbon connection, the workhorse of the molecular world. It’s probably in more things than you can even imagine, from the plastic in your phone case to the sugar in your coffee.
Then, things get a little more exciting with double bonds. This is like a firm, confident handshake, maybe with a little enthusiastic side-to-side shake thrown in. Here, two carbon atoms are sharing two pairs of electrons. It's a bit more of a commitment, a tighter connection. You see these a lot in things like vegetable oils, where the double bonds make the molecules a bit more flexible and prone to going rancid if left out too long. It’s like that friend who’s always a little too enthusiastic about everything – sometimes it’s great, sometimes it’s a bit much.
And finally, the superstar of our show, the triple bond! This is the molecular equivalent of a power hug, a really, really tight embrace. Two carbon atoms are sharing three pairs of electrons. They are practically fused together, holding on for dear life! This is where things get seriously short. Think of it as the ultimate in molecular intimacy, a bond so strong and so close, it’s practically a single, unified entity.
So, if triple bonds are the tightest, most intimate connections, it stands to reason that they're going to have the shortest bond lengths, right? It’s like when you’re trying to squeeze into a tight parking spot – the more "effort" you put in (more shared electrons), the closer you get to the curb. And when it comes to carbon-carbon bonds, the triple bond is the undisputed parking champion.
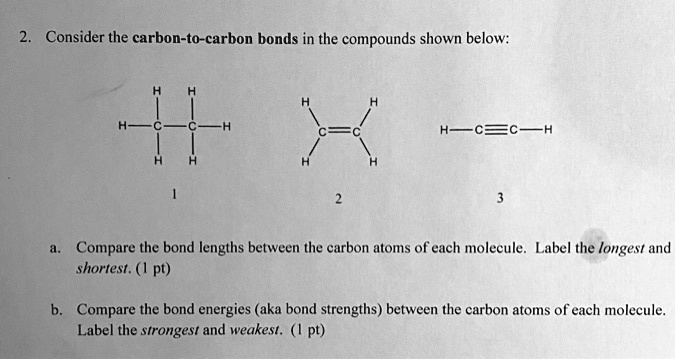
Let's talk numbers, because even though they're tiny, they tell a story. A typical carbon-carbon single bond, in a molecule like ethane (two carbons with a single bond), is around 1.54 angstroms. That's our relaxed handshake. Not too shabby, but definitely not breaking any speed records for closeness.
Now, dial it up to a carbon-carbon double bond, found in a molecule like ethene. This bond length shrinks to about 1.34 angstroms. See? It’s already gotten a bit more snug, a bit more serious. It’s like going from a casual wave to a proper high-five. More energy, more connection.
But here's where the magic happens. When we get to the carbon-carbon triple bond, the bond length plummets to an astonishingly short 1.20 angstroms! That's a significant jump in closeness. Imagine going from a polite nod to a full-on, "I haven't seen you in ages!" hug. The difference is palpable, even in the microscopic world.
So, the undisputed champion, the holder of the shortest carbon-carbon bond length, is the carbon-carbon triple bond. You'll find this super-tight connection in molecules like acetylene (also known as ethyne), the stuff they use in welding torches. Talk about a strong bond!

Why is it so short? It all comes down to electron density and orbital overlap. Think of those electrons as little magnets. When you have more pairs of electrons trying to bond, they get squeezed together, pulling the two carbon atoms closer and closer. It’s like trying to get two magnets to repel each other – the more poles you try to force together, the harder they push, but in this case, they're attracting! It's a delicate dance of forces.
Also, the hybridization of the carbon atoms plays a role. In a triple bond, each carbon atom is 'sp hybridized'. This means their electron orbitals are more linear and elongated, allowing for better overlap with the other carbon atom’s orbitals. It’s like having perfectly shaped LEGO bricks that just snap together with minimal wiggle room, as opposed to round marbles that can roll away easily.
Imagine you're trying to fit two pieces of a puzzle together. If they have a single, simple connection, they might have a bit of give. If they have a double connection, they're more locked in. But if they have a triple connection, with multiple interlocking tabs and slots, they are going to fit together very snugly. That's essentially what's happening with the carbon atoms and their electrons in a triple bond.
This extreme closeness and strength of the triple bond has some really cool consequences. Molecules with triple bonds are often more reactive. That tight grip makes them eager to share those electrons with other molecules, leading to interesting chemical reactions. Think of it as having a lot of pent-up energy that’s just waiting to be unleashed. It’s like that friend who’s so excited about a surprise party, they can barely sit still!
The most famous example of a carbon-carbon triple bond is in acetylene (C₂H₂)**. This molecule is incredibly simple – just two carbon atoms and two hydrogen atoms. But that triple bond between the carbons is the star of the show. It’s what gives acetylene its intense heat when burned, making it invaluable for welding and cutting metal. So, the next time you see that brilliant blue flame, remember the tiny, incredibly short triple bond working its magic!
It's also found in other important molecules. For instance, nitriles, which have a -C≡N functional group, have a carbon-nitrogen triple bond. While we're focusing on carbon-carbon here, it’s a good reminder of how potent triple bonds can be. And in the realm of organic chemistry, where carbon is king, the C≡C triple bond is a real power player.
You might also encounter triple bonds in things like alkynes. These are hydrocarbons that contain at least one carbon-carbon triple bond. They're a whole class of compounds, and their triple bonds dictate a lot of their behavior. They're like the edgy, rockstar cousins of the more laid-back alkanes (with single bonds) and alkenes (with double bonds).
So, to recap our journey into the minuscule world of molecular distances: we’ve learned that the shortest carbon-carbon bond length belongs to the carbon-carbon triple bond. It's a testament to the power of sharing electrons, a tight embrace that pulls these atoms closer than any other carbon-carbon connection. It’s shorter than a double bond, and a whole lot shorter than a single bond. It’s the ultimate in molecular closeness.
Think of it like this: a single bond is like a polite wave. A double bond is a firm handshake. And a triple bond? That’s a full-on, enthusiastic hug where you can barely breathe because you're squeezed so tight. That's the kind of closeness we're talking about!
It's amazing to think that these incredibly small differences in bond length can lead to such profound differences in a molecule's properties, from its stability to its reactivity. It’s like how a slight change in your driving can take you to a completely different destination. Tiny adjustments, huge outcomes.
So, the next time you're munching on some organic goodness, or even just admiring a piece of metal art that was welded, take a moment to appreciate the incredible work of those tiny, powerful carbon-carbon triple bonds. They might be invisible, they might be incredibly short, but they are undeniably significant. They are the champions of closeness in the carbon-carbon world, proving that sometimes, the strongest connections are also the shortest!
It’s a bit like life, isn’t it? Sometimes the most profound relationships, the ones that really matter, are the ones that are incredibly intense and close. Not necessarily long and drawn out, but deeply connected. And in the molecular world, that depth of connection is measured in angstroms and the number of shared electron pairs. Who knew chemistry could be so relatable?
So, there you have it. The shortest carbon-carbon bond? It's the triple bond, hands down. It's the molecular equivalent of a super-tight hug, a testament to the power of sharing. Keep an eye out for those triple bonds; they're the little powerhouses of the chemical world, packing a punch (and a very short bond length)!
