Which Compound Does H2so4 Represent When In An Aqueous Solution

Hey there, ever find yourself staring at a bottle of something that looks suspiciously like battery juice and wondering, "What in the heck is that stuff?" We're talking about H₂SO₄, folks, or as us mere mortals know it, sulfuric acid. Now, before your mind conjures up images of mad scientists cackling and bubbling beakers of doom, let's take a deep breath and get cozy with this often-misunderstood chemical. It's not all doom and gloom, and believe it or not, you've probably had a run-in with its aqueous form more times than you'd think.
Think of it like this: you know how some people are really intense in their pure, undiluted form? Like that one friend who only talks about their obscure hobby, and if you don't know what a "furlong" is, you're basically lost at sea. Yeah, that's sulfuric acid in its concentrated state. It's powerful, it's serious business, and it's best admired from a safe distance, preferably with really good safety goggles and a hazmat suit on standby. But then, there are times when even the most intense personalities mellow out. They become more approachable, more integrated into the social scene. They might still have their core essence, but they're not going to bite your head off for asking a simple question.
That's precisely what happens when H₂SO₄ decides to go for a dip in water. It's like it's saying, "Okay, okay, I get it. I don't need to be that intense all the time." When sulfuric acid meets water, it doesn't just sit there looking pretty. Oh no, it gets a bit… excited. It’s like throwing a pop-rock into a glass of soda. There's a bit of fizz, a bit of reaction, and a whole lot of change happening. And this, my friends, is where we get to the heart of the matter: what compound does H₂SO₄ represent when in an aqueous solution?
The Great Dissolving Act
When sulfuric acid (H₂SO₄) hits the water party, it doesn't stay as a polite H₂SO₄ molecule. It's more like a drama queen who, upon entering a room, immediately starts making a scene – a good scene, in this case! It’s a bit like when you're making lemonade. You don't just dump a whole lemon in water and expect a refreshing drink. You squeeze that lemon, and the juice, the essence of the lemon, mixes and disperses. Sulfuric acid does something similar, but with a lot more gusto and a bit more… ionization.
Essentially, the H₂SO₄ molecule is like a parent with two eager children, the hydrogen ions (H⁺). When the water molecules come around, they're like a welcoming committee. They surround the sulfuric acid, and in a glorious act of chemical emancipation, the sulfuric acid splits apart. It loses one, and sometimes even both, of its hydrogen ions. This process is called dissociation, or more specifically in this case, ionization. It’s like a balloon that bursts, scattering its colorful pieces everywhere.
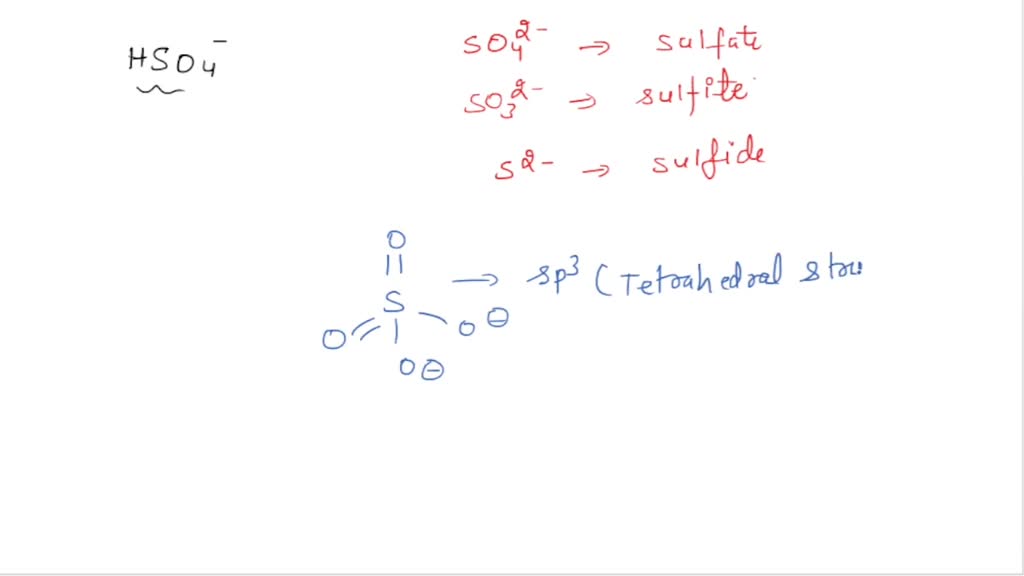
So, instead of a lone H₂SO₄ molecule, you end up with a bustling crowd of charged particles. You'll find hydrogen ions (H⁺) zipping around, feeling all excited and positive. And you'll also have the remaining part of the sulfuric acid molecule, which is now a bisulfate ion (HSO₄⁻) or, if it's really gone all out, a sulfate ion (SO₄²⁻). These guys are the slightly more reserved cousins who are still happy to be part of the party, just not quite as energetic as the H⁺ ions.
The Main Players in the Aqueous Arena
So, when you see H₂SO₄ in water, it’s not just H₂SO₄ anymore. It’s a whole gang. The primary players you’ll be seeing are:
- Hydrogen Ions (H⁺): These are the little dynamos. Think of them as the life of the party, always on the move and contributing to the overall vibe. They're responsible for making the solution acidic.
- Bisulfate Ions (HSO₄⁻): This is the H₂SO₄ molecule that’s given up just one of its hydrogen kids. It's still got a bit of that acidic punch, but it's slightly less intense than a pure hydrogen ion.
- Sulfate Ions (SO₄²⁻): This is the H₂SO₄ molecule that’s completely let go of both its hydrogen children. It’s the calmest of the bunch, just chilling in the water.
The beauty of an aqueous solution of sulfuric acid is that it's a dynamic mix. These ions aren't just sitting there; they're constantly interacting with the water molecules, and with each other. It’s like a busy city street where cars (ions) are constantly moving and interacting.
Why Should You Care About This Chemical Shindig?
Now, you might be thinking, "Okay, chemical jargon, yay. But why is this relevant to my Tuesday afternoon?" Well, this whole ionization thing is actually super important. It’s what gives sulfuric acid its signature properties. Because of all those zippy hydrogen ions, aqueous sulfuric acid is a potent acid. It’s the king of acids, in fact, often referred to as the "king of chemicals" for its widespread use.

Think about your own digestive system. Your stomach acid, which helps break down your food, is primarily hydrochloric acid, but it’s still an acid, right? It’s got those H⁺ ions doing their job. Sulfuric acid, when diluted, acts in a similar way – it can donate those H⁺ ions to other substances, causing reactions. It’s like a really helpful, albeit powerful, friend who’s always ready to lend a hand (or an ion).
You encounter the effects of this aqueous magic more than you might realize. Ever used a car battery? Yep, the electrolyte inside is essentially a dilute solution of sulfuric acid. It’s this ability to release and accept ions that allows the battery to store and release electrical energy. It's like the battery's tiny internal engine, powered by these charged particles.
And what about fertilizers? Many of them contain sulfates, which are derived from sulfuric acid. These sulfates are essential nutrients for plants, helping them grow big and strong. So, the next time you see a lush green lawn or a bountiful harvest, you can nod your head and think, "Ah, aqueous sulfuric acid, you’re playing your part!"
Even in things like laundry detergents, you might find sulfates. They help lift dirt and grime. It’s like having a tiny army of cleaning agents working away in your washing machine, thanks to the properties that sulfuric acid brings to the table when it’s hanging out in water.

The Not-So-Scary Side of Sulfuric Acid
The key word here is aqueous solution. It’s the dilution that makes all the difference. Concentrated sulfuric acid is a beast. It's a dehydrating agent (meaning it sucks the water right out of things – think of it as a super-powered sponge for molecules) and can cause severe burns. It’s the kind of stuff you definitely want to handle with extreme caution.
But when it’s mixed with water, it’s like a tamed lion. It’s still strong, and it demands respect, but it’s not going to go on a rampage and eat your furniture. It becomes a useful tool. Imagine a really strong coffee. In its concentrated espresso form, it can be a bit overwhelming. But when you add milk and sugar (like water for sulfuric acid), you get a delicious latte that’s enjoyable and invigorating. That’s the aqueous solution for you.
The concentration of the sulfuric acid in the water is what determines how potent it is. A highly concentrated solution will have more ions, making it a stronger acid. A very dilute solution will have fewer ions, and while it's still acidic, it's much less aggressive. It’s like the difference between a gentle drizzle and a full-blown thunderstorm. Both are rain, but one is a lot more intense.

So, What Does H₂SO₄ Represent?
Ultimately, when H₂SO₄ is in an aqueous solution, it represents a powerful source of hydrogen ions and sulfate/bisulfate ions. It’s not just one single compound anymore; it’s a dynamic mixture that exhibits acidic properties. It’s the chemical equivalent of someone who’s incredibly talented but also knows how to collaborate and share their talents with others.
It’s the reason why that battery works, why fertilizers help plants grow, and why certain cleaning products can tackle tough grime. It’s a chemical workhorse, and its ability to break apart and form these ions in water is what makes it so incredibly versatile and indispensable in so many aspects of our modern lives.
So, the next time you hear about sulfuric acid, don't just picture a dangerous chemical. Remember the aqueous solution, the diluted, approachable, yet still powerfully effective form. It’s a testament to how things can change and become incredibly useful when they find the right environment – in this case, a nice, comforting splash of water. It's like a celebrity who, off the red carpet, is just a regular person, albeit a very impactful one!
It’s a reminder that even the most formidable substances can be harnessed for good when understood and applied correctly. And that, my friends, is the everyday magic of chemistry, right there in your car battery and your lawn.
