Which Class Of Organic Compound Would Neutralize A Carboxylic Acid
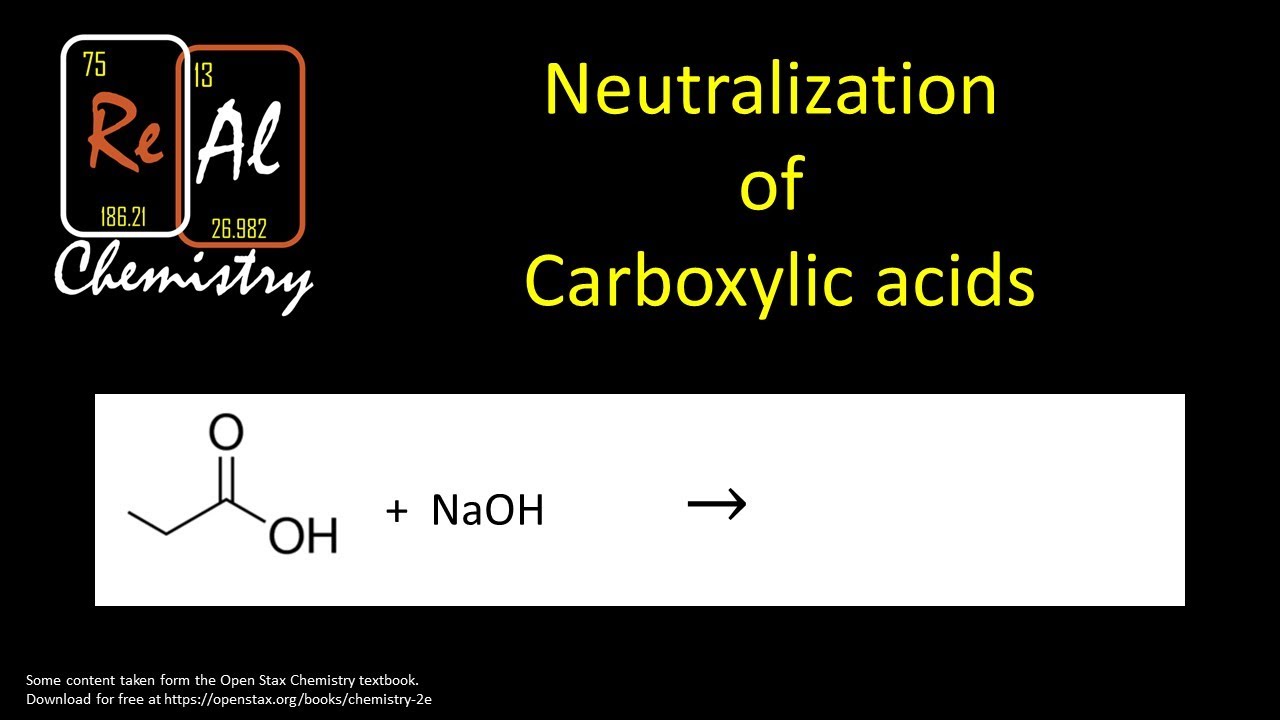
Ever found yourself staring into the chaotic abyss of your kitchen, wondering which of those mysterious bottles lurking in the back could perform a chemical rescue mission? Well, hold onto your spatulas, folks, because we're about to embark on a thrilling expedition into the world of organic chemistry, where even the most mundane ingredients can become superheroes!
Imagine this: your car is sputtering, making funny noises, and the check engine light is flashing like a disco ball gone rogue. What do you do? You don't grab a bag of sugar, right? You need something with a bit more oomph, something designed to fix that specific kind of engine trouble. The same principle applies in the microscopic world of molecules!
Today, we're focusing on a particular type of molecule that can sometimes be a little too enthusiastic, a little too acidic. Think of it like a cranky old grandpa who's had a bit too much caffeine. These are our carboxylic acids. They’ve got that "COOH" thing going on, which basically means they're ready to donate a tiny particle, making them a bit sour and reactive.
Now, what in the vast universe of bubbly liquids and powdery delights can tame this acid's fiery spirit? What can bring peace to the molecular kingdom? Drumroll, please...
The Unsung Heroes of Neutralization!
Prepare yourselves, for we are about to meet the champions, the peacemakers, the ones who say, "Whoa there, little acid, let's calm down!" These are the amazing bases! Specifically, we're talking about organic bases, which have a special talent for making things right when acids go a little too wild.
Think of a really strong glass of lemonade. It's tart, right? That tartness is thanks to acids. Now, imagine adding a tiny bit of baking soda to it. Poof! The fizzing stops, and the lemonade tastes much less sour. That baking soda, in its own humble way, is acting like our hero base, neutralizing the excess tartness.
In the grand theater of chemistry, carboxylic acids are often the dramatic ones, prone to oversharing their acidity. They can be found in everything from the tang of vinegar to the zing of a lemon.
+group..jpg)
But fear not! There's always a solution. And in the case of carboxylic acids, that solution often comes in the form of an amine. Yes, you heard me right! Amines are these super cool organic compounds that are practically begging to be friends with our acidic pals.
Enter the Amazing Amines!
So, what exactly is an amine? Imagine a nitrogen atom that's decided to make some friends. It's usually hanging out with some hydrogen atoms, but in amines, it's also formed bonds with some of our good ol' carbon chains. This makes them a little bit basic, like a friendly hug for an overly excited molecule.
The simplest amine you might have heard of is ammonia. You know, that stuff that can sometimes have a pungent smell? While ammonia itself is inorganic, it's the grandparent of many organic amines. It’s like the wise old elder of the amine family.
Now, when an amine meets a carboxylic acid, it's like a perfectly choreographed dance. The amine, with its welcoming embrace (thanks to that nitrogen atom!), swoops in and kindly accepts the little particle that the carboxylic acid is so eager to give away.
This donation-and-acceptance process is called a neutralization reaction. It’s where the acid’s sourness and the base’s bounciness cancel each other out, leaving behind something much more mellow. It’s like a tiny chemical truce!
Think about it like this: if a carboxylic acid is a really loud person at a party, constantly shouting about how acidic they are, an amine is the cool, calm friend who gently puts a hand on their shoulder and says, "Hey, let's chill for a bit." The shouting (acidity) stops, and everyone can relax.
One of the most common and fun examples of an amine is ethylamine. It's like the energetic younger sibling of ammonia, always ready for action. When ethylamine encounters a carboxylic acid, it’s a match made in molecular heaven.
The nitrogen atom in ethylamine is like a little molecular magnet, positively charged and ready to attract the negatively charged part of the carboxylic acid. It’s a beautiful chemical attraction!
This reaction creates what we call a salt. Not the kind you sprinkle on your fries, though. This is a special chemical salt, formed from the union of our acid and base. It's like their happily-ever-after in the chemical world!

Imagine a fussy chef who's made a sauce that's a little too vinegary. They might add a pinch of baking soda to mellow it out. In the organic world, our amines are doing a similar job, but on a much smaller, molecular scale. They’re the secret ingredient to making things just right!
So, the next time you hear about a carboxylic acid causing a stir with its acidity, you can smile and know that there's a whole army of fabulous amines ready to come to the rescue. They are the ultimate calmers, the perfect neutralizers, and the unsung heroes of many a chemical reaction.
It's like having a superhero team! The carboxylic acid is the one with the dramatic powers, maybe a bit too much energy. And the amine? That's the one with the super-soothing touch, bringing everything back to balance. They're the dynamic duo of the molecular world!
These reactions are happening all around us, in our bodies, in nature, and even in the food we eat. And it’s all thanks to the amazing properties of compounds like amines.
So, to answer the burning question: which class of organic compound would neutralize a carboxylic acid? It's the ever-so-charming and wonderfully basic amines! They’re the perfect antidote to acidic shenanigans, making the world a more balanced and harmonious place, one molecule at a time.
Next time you're dealing with something a bit too tart or sour, just remember the power of a good amine. They’re the true MVPs of neutralization, and they deserve a round of applause – or at least a cheerful molecular wave!
And remember, while we're talking about everyday examples, the chemical reactions in laboratories can be far more complex. But the fundamental principle of an acid meeting a base to neutralize each other remains a constant, beautiful truth in the vast universe of chemistry. It’s a testament to the order and balance that exists even in the tiniest of worlds.
So, there you have it! The mystery is solved, and the heroes are revealed. It's a delightful little peek into how the world of molecules works, and it's all thanks to the incredible properties of amines and their ability to bring peace to the feisty world of carboxylic acids.
Keep exploring, keep questioning, and most importantly, keep enjoying the fascinating journey of discovery. Who knows what other molecular marvels await your curious gaze!
