Which Characteristic Do Valence Electrons Indicate About Reactions Between Atoms
Hey there! Grab a coffee, pull up a chair. We’re gonna chat about something super cool today, something that basically explains everything when it comes to how atoms decide to hang out, or, you know, not hang out. We're diving into the world of valence electrons. Sounds fancy, right? But honestly, it’s like the atoms' dating profiles. They tell you everything you need to know.
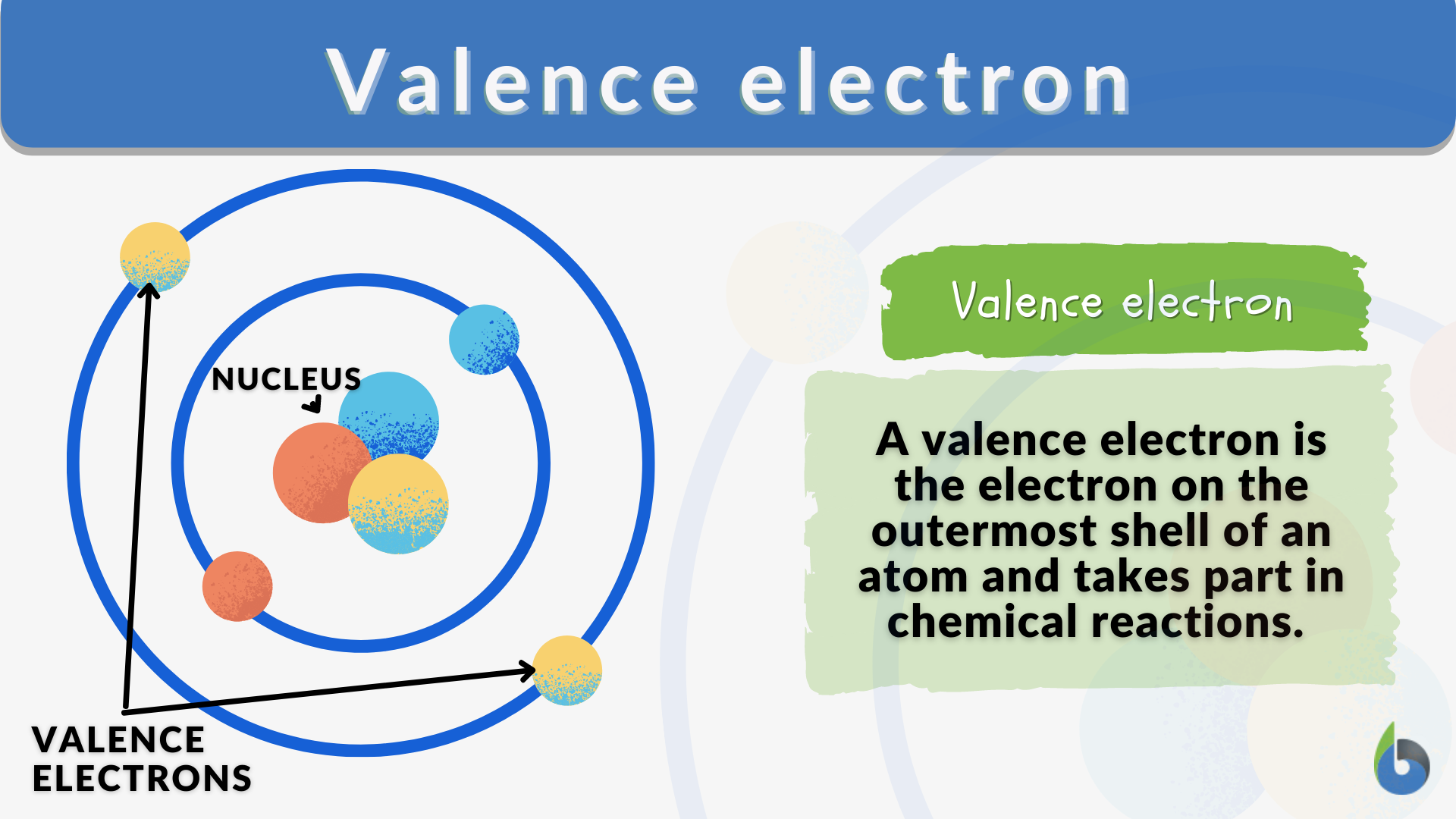
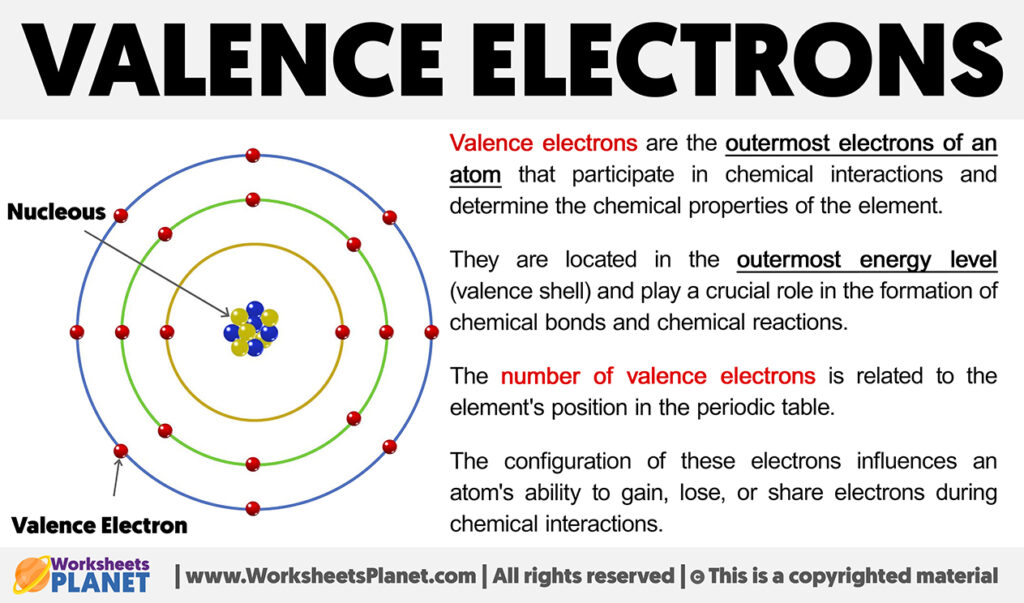
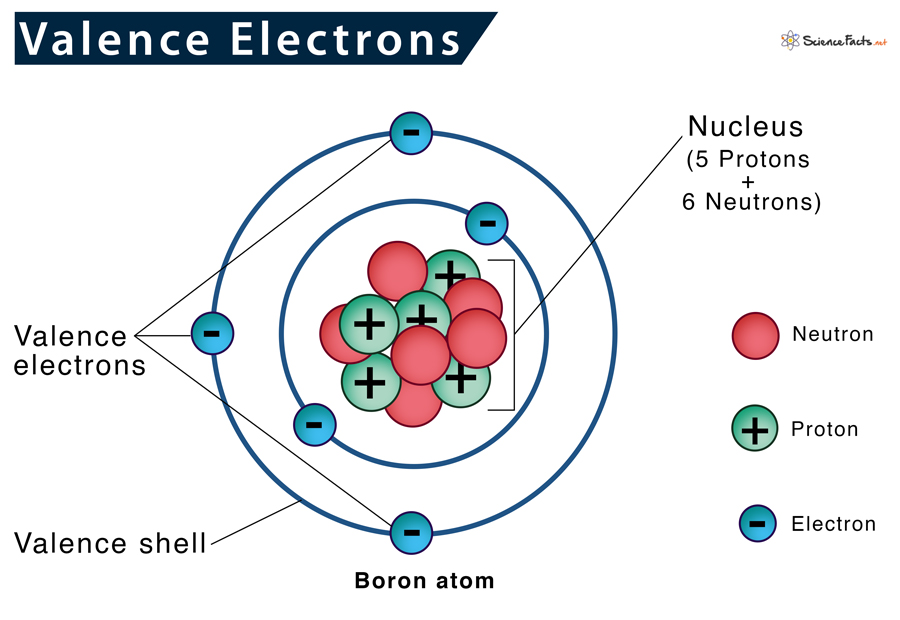
So, what are these mysterious valence electrons anyway? Think of an atom like a tiny apartment building. You’ve got these little electron dudes zipping around the nucleus, which is like the building’s super-fancy lobby. These electrons live on different floors, or energy levels. The valence electrons? They’re the ones living on the outermost floor. The penthouse suite, if you will. And trust me, they are the most important residents.
Why so important, you ask? Because they’re the ones who get to go out and mingle. The inner electrons? They're busy with their own stuff, maybe watching atom-TV or something. But the valence electrons? They’re the ones sticking their heads out the window, looking for neighbors. They’re the social butterflies of the atomic world.
And that, my friends, is where the magic happens. These valence electrons are the ones who decide if two atoms are going to get along, become best buds, or just completely ignore each other. It’s all about them. They’re the ultimate matchmakers.
The Grand Plan: Stability!
Atoms, bless their little electron-filled hearts, have one primary goal in life: stability. They want to feel complete, content, like they've finally found their missing sock. And for most atoms, this means having a full outer shell of electrons. It’s like wanting to finish a puzzle, you know? You just feel better when all the pieces are in place.
For many atoms, especially those in the first few rows of the periodic table (which, by the way, is basically a giant cheat sheet for atom behavior!), having eight electrons in their outermost shell is the golden ticket to happiness. This is called the octet rule. Eight's the magic number, folks. It’s like the atomic equivalent of a perfectly balanced meal.
Think about it. If an atom has, say, seven valence electrons, it’s one electron short of that perfect eight. It's perpetually feeling that slight itch, that "almost there" vibe. So what does it do? It goes looking for someone who has an extra electron to spare, or someone it can take an electron from. Desperate times, right?
On the flip side, what if an atom has just one valence electron? Oh boy. That’s like having way too much pizza at a party. You’re just eager to get rid of it, to share the love, or maybe just lighten the load. So, an atom with one valence electron is super eager to donate that electron. It's practically handing it over with a smile.
The Great Electron Exchange: Bonding!
This whole dance of gaining, losing, or sharing electrons is what we call chemical bonding. And it's all thanks to our valence electrons. They're the currency of the chemical world!
So, how does this play out? Let’s imagine two atoms chatting. Atom A has seven valence electrons, practically begging for one more. Atom B has just one valence electron, practically bursting to give it away. What do you think happens?
Yup. They’re a match made in chemical heaven! Atom B happily gives its electron to Atom A. Now, Atom A has a full outer shell of eight. Hooray! And Atom B? Well, its previous outer shell (which is now its outermost shell) is also full. Double hooray! They’ve both reached that blissful state of stability. And guess what? They now stick together. They’ve formed a chemical bond. It’s like they’ve decided to move in together, a little atomic cohabitation.
This is the basis of ionic bonding. One atom loses an electron, becoming positively charged (it’s lost a negative charge, so it’s now got more positives hanging around). This is called a cation. The other atom gains the electron, becoming negatively charged. This is an anion. And what do opposite charges do? They attract! Like tiny atomic magnets. Boom! Ionic bond.
Think of salt, NaCl. Sodium (Na) has one valence electron. Chlorine (Cl) has seven. Sodium is like, "Here, take it!" Chlorine is like, "Yes, please!" Sodium becomes Na+ and Chlorine becomes Cl-. They’re now attracted to each other, forming the salt crystals you sprinkle on your fries. Delicious and stable!

Sharing is Caring: Covalent Bonds
But what if neither atom is in a hurry to completely give away or take an electron? What if they’re both a little bit… indecisive? Or maybe they’re both really keen on holding onto their own electrons, but still want to feel that satisfying full shell?
This is where the next flavor of bonding comes in: covalent bonding. And this is where valence electrons really show their collaborative spirit. Instead of an outright transfer, these atoms decide to share their valence electrons.
Imagine two atoms, each with, say, six valence electrons. They’re both two electrons short of that magic eight. Neither wants to give up their six, but they both desperately need two more. So, they come to an agreement: "Hey, why don't we each lend you two of my electrons, and you lend me two of yours? We’ll call it a joint venture!"
They then hover their valence electrons in the space between them, like a little electron playground. These shared electrons now orbit both nuclei, effectively giving each atom the satisfaction of a full outer shell. It's like a potluck dinner for electrons – everyone brings something, and everyone gets to enjoy the whole spread.
Water (H2O) is a classic example of covalent bonding. Oxygen has six valence electrons. Hydrogen has one. Oxygen needs two more, and each Hydrogen needs one more. So, Oxygen shares one of its electrons with each Hydrogen atom, and each Hydrogen atom shares its electron with Oxygen. Voila! A stable water molecule. Pretty neat, huh?

The Spectrum of Reactivity
Now, not all atoms are created equal when it comes to their valence electrons. Some have very few, some have almost a full house. This directly impacts how reactive they are.
Atoms with just one or two valence electrons? They're like the eager beavers of the chemical world. They are super reactive. They’re practically jumping at the chance to get rid of those extra electrons and achieve stability. Think alkali metals like Sodium (Na) and Potassium (K). They’re famous for their explosive personalities, literally!
Atoms with six or seven valence electrons? They’re also highly reactive, but in a slightly different way. They’re the ones looking to grab those missing electrons. They’re the electron magnets. Halogens like Fluorine (F) and Chlorine (Cl) are in this category. They’re eager to pair up with those one- or two-electron atoms.
And then you have the rockstars, the divas, the ones who are already perfectly content. I’m talking about the noble gases. Think Helium (He), Neon (Ne), Argon (Ar). These guys have a full outer shell of electrons. They’re already stable. They’ve achieved atomic nirvana. Because of this, they are incredibly inert, meaning they hardly react with anything. They’re the loners, the ones who are perfectly happy in their own little electron bubbles. They’re the introverts of the periodic table, and honestly, who can blame them when they’re already that content?
More Than Just a Number
So, you see, it's not just about how many valence electrons an atom has. It's also about where they are in their quest for stability.
If an atom has just one electron, it's going to be much more inclined to lose it. If it has seven, it's going to be much more inclined to gain one. These tendencies dictate the types of bonds they form and, consequently, the kinds of compounds they create.
And it’s not just about simple gains and losses. Sometimes, atoms with a few valence electrons might prefer to share rather than give them up entirely. The number of valence electrons tells us their potential for either donating, accepting, or sharing, and that’s a huge clue about their chemical personality.
It's all about striving for that perfect, happy, full outer shell. And the valence electrons are the willing participants in this grand atomic quest. They are the ultimate indicators of an atom's desire to interact, to change, to become something more.
Seriously, the next time you look at something – a glass of water, a rock, a piece of metal – remember that it’s all happening because of these tiny, energetic little dudes on the outside of atoms. The valence electrons! They’re the true movers and shakers of the universe. They’re the reason why things stick together, why reactions happen, and why the world around us is so incredibly diverse. So, a little applause for the valence electrons, please! They’re doing all the heavy lifting (or, should I say, the electron-lifting) for us.
And that, my friend, is the long and short of it. Valence electrons: they’re not just a concept in a textbook, they are the absolute heart of chemical reactions. Pretty mind-blowing when you think about it, right? Now, who needs a refill?
