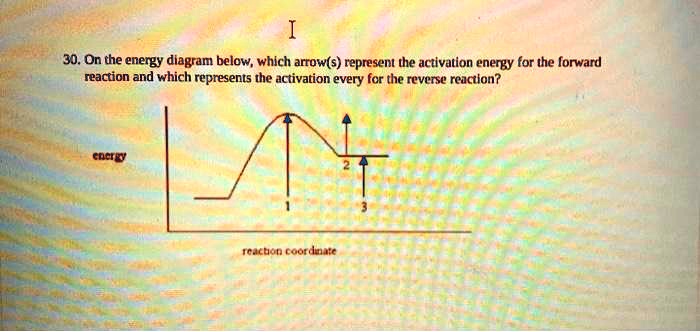
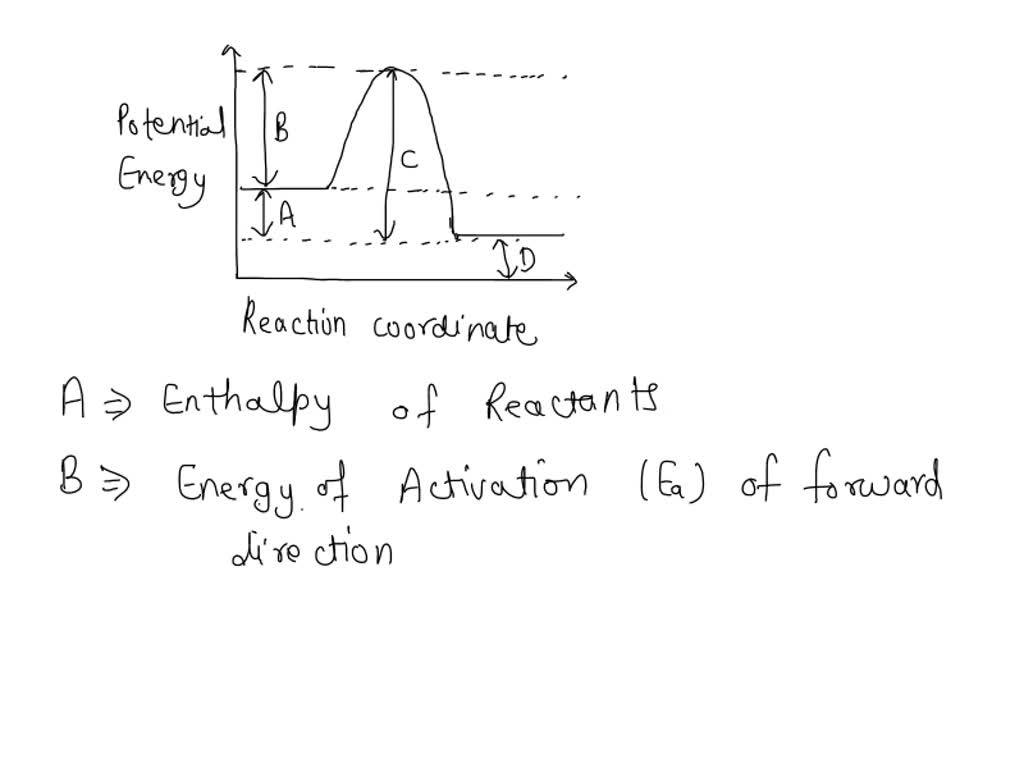
Which Arrow Represents The Activation Energy Of The Forward Reaction

Ever wondered what makes a chemical reaction go? It's like a tiny, invisible hurdle race for molecules. And in this race, one arrow in particular is a real superstar. This arrow is the one that shows us the Activation Energy of the forward reaction.
Imagine you're trying to push a boulder up a hill. That hill? That's the activation energy. It's the initial push, the extra oomph, that your molecules need to get started.
Without this starting boost, even if the reaction wants to happen, it just won't. It's like trying to start a car without a key. You've got all the potential, but you need that spark!
So, when you see a diagram showing a chemical reaction, look for that special arrow. It's usually pointing upwards, representing that climb. It's the visual cue for the energy barrier that needs to be overcome.
This arrow isn't just a random squiggle on a page. It tells a whole story about how easily or difficultly a reaction can get going. A tall arrow means a big hill to climb, and a lot of energy needed. A short arrow means a smaller hill, and an easier start.
Think of it like baking. You need to preheat your oven, right? That's a kind of activation energy. You're giving the ingredients the energy they need to transform into delicious cookies.
In the world of chemistry, this activation energy arrow is like the "go" signal. It's what allows the molecules to break old bonds and form new ones. It's the key that unlocks the chemical change.
The arrow representing the Activation Energy for the forward reaction is particularly exciting. Why? Because it's the first step in our molecular adventure! It’s the start of the main event.
This arrow shows us the minimum amount of energy required to get the molecules moving in the desired direction. It’s the difference between a "maybe" reaction and a "definitely happening" reaction.
Scientists use these diagrams to understand how reactions work. They can see how changing conditions, like adding a catalyst, can affect the height of that energy hill. And that's super cool!
A catalyst is like a helpful friend who gives you a boost up the hill. It lowers the activation energy, making the reaction happen faster and with less effort. The arrow representing the catalyzed reaction would be noticeably shorter!

So, that single arrow for forward activation energy is more than just a graphic. It's a window into the very heart of chemical transformations. It's the point where possibilities turn into realities.
It's like a tiny gatekeeper. Only if the molecules have enough energy can they pass through and become something new. This is where the magic begins, and that arrow is its herald.
Sometimes, you'll see this arrow drawn very clearly on energy profile diagrams. It's usually a distinct line, soaring upwards. It’s the visual embodiment of effort and potential.
This concept of activation energy is what makes chemistry so fascinating. It’s not just about mixing things together; it’s about understanding the energy dynamics involved. And that arrow is our guide.
When we talk about the Activation Energy of the forward reaction, we're talking about the initial investment. It's the energy cost to get the ball rolling on the path to products.
Consider a tiny spark igniting a much larger fire. That spark is the activation energy. It’s the small thing that unleashes a big change. The arrow shows us just how big that spark needs to be.
It’s a beautiful illustration of how even the smallest energetic push can lead to significant outcomes. It highlights the delicate balance in chemical processes.
This arrow can also tell us about the stability of the reactants. If the activation energy is very high, the reactants are quite stable and don’t readily transform.
Conversely, a low activation energy means the reactants are more eager to react. They don't need much convincing, or much of a push up that hill.
The arrow is a visual representation of that "convincing." It's the minimum energy needed to persuade those molecules to change their minds and rearrange themselves.
What makes it so entertaining is the way it simplifies a complex idea. Instead of long, complicated explanations, we have a clear, visual cue. It’s chemistry made digestible and intriguing.
This arrow is the star of the show when we're discussing how reactions begin. It's the point of no return, in a way. Once past that energy hurdle, the reaction is well on its way.
It’s like the starting pistol in a race. All the runners are lined up, but they can’t move until that bang. The activation energy is that bang for molecules.
And the arrow is the visual cue for that bang. It shows us the intensity of that initial signal. It’s a subtle yet powerful detail.
When you see that arrow, think about the energy it represents. Think about the effort molecules must exert. It’s a tiny, epic struggle happening all around us!
The Activation Energy arrow is a fundamental concept, but it's also one that sparks curiosity. It makes you want to learn more about what’s happening at the molecular level.
It’s the promise of transformation. The hint of what can be achieved with just the right amount of energy.
So, next time you encounter a diagram depicting a chemical reaction, keep an eye out for that special arrow. The one that points to the Activation Energy of the forward reaction.
It’s a simple yet profound symbol. It’s the key to understanding the energetic beginnings of every chemical change. And that’s truly something to be excited about!
It’s like the first domino falling in a long chain. That initial push, represented by the arrow, sets off a cascade of events.
This concept makes chemistry feel more alive, more dynamic. It’s not static; it’s a constant dance of energy and transformation.
The arrow is a testament to the fact that even the smallest energetic inputs can have monumental consequences. It’s a lesson in the power of a well-timed push.
What’s special about it is its universality. This concept applies to countless reactions, from the simplest to the most complex.
It's a common language that chemists use worldwide to describe reaction kinetics. It’s a shared understanding of molecular hurdles.
And that arrow is the visual shorthand for this crucial idea. It’s efficient, elegant, and incredibly informative.
It’s the reason why some things happen instantly, and others take ages. It’s all about the height of that molecular hill.
So, embrace the mystery and the elegance of this single arrow. It’s a tiny symbol with a huge story to tell about the world of chemical reactions.

It’s an invitation to explore further, to delve deeper into the fascinating world of chemistry. And who knows what other exciting concepts you might discover!
This arrow is the unsung hero of many chemical explanations. It’s the silent narrator of the reaction’s journey from start to finish.
It’s a reminder that even in the smallest scales, there’s an inherent energy at play, driving change and evolution. It’s a tiny force with enormous implications.
So, let that arrow intrigue you. Let it spark your imagination. It’s the gateway to understanding the dynamic and energetic world of chemistry.
It’s the visual representation of potential energy waiting to be unleashed. The promise of what lies beyond the initial barrier.
Ultimately, the arrow representing the Activation Energy of the forward reaction is a beautiful and accessible concept. It’s a fantastic starting point for anyone curious about the magic of chemistry.
It makes complex processes understandable and visually engaging. It’s like a secret code that unlocks deeper knowledge.
And that, in itself, is quite special, wouldn't you agree?
