When The Reaction Produces No Intermediates Which Of The Following
Hey there, science explorers! Ever stop to think about how things change around us? You know, like when baking a cake, or when a leaf turns from green to fiery red in the fall. Those are chemical reactions, and they're happening all the time, even when we're not looking.
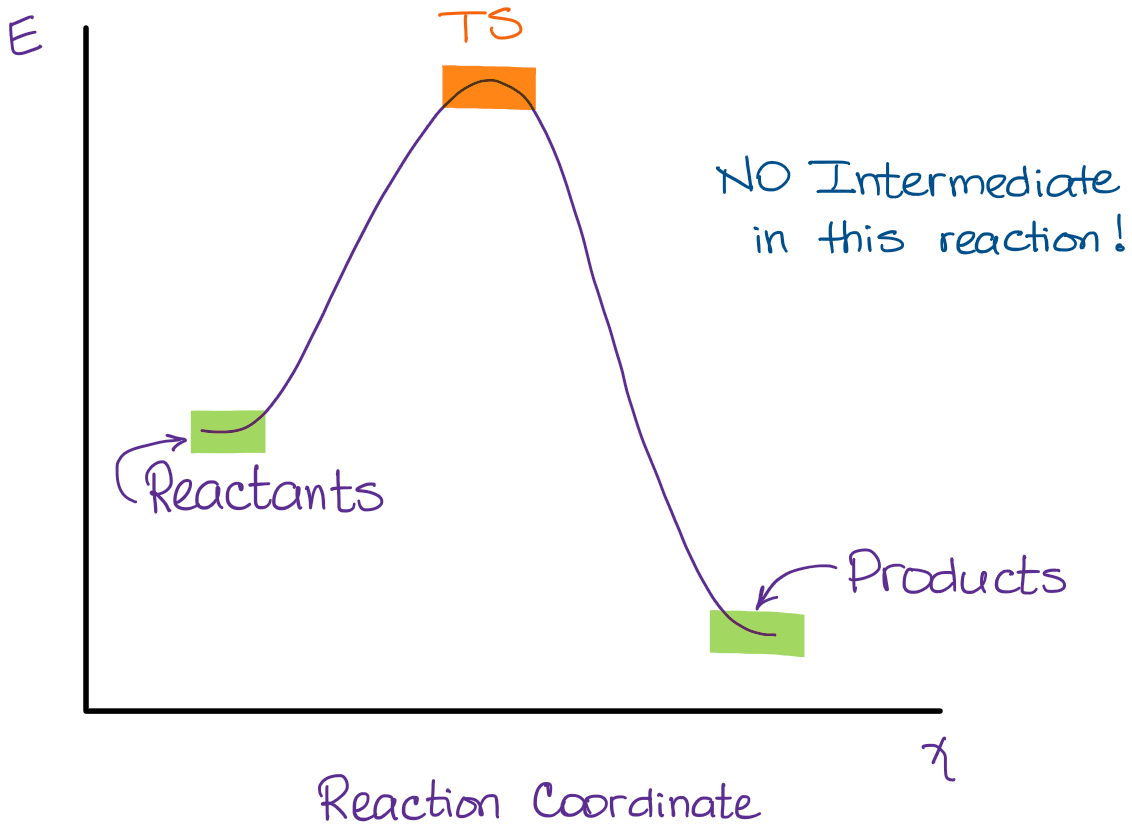
But have you ever wondered about the journey a reaction takes? It's not always a straight shot from start to finish. Sometimes, it's more like a winding road with a few pit stops along the way. Today, we're going to peek into one of those "straight shot" scenarios. We're talking about when a chemical reaction produces no intermediates. Sounds a little mysterious, right?
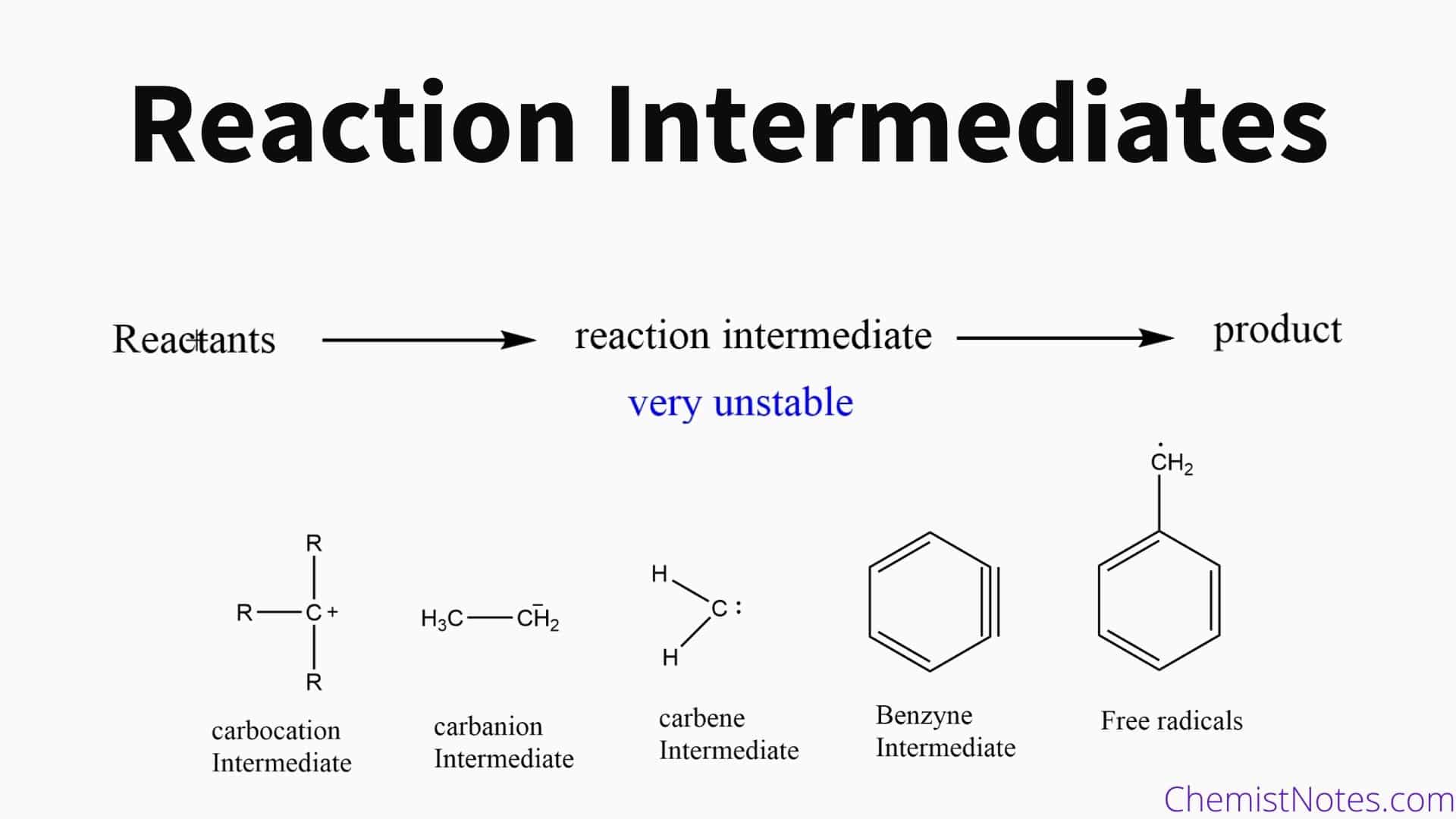
So, what exactly are these "intermediates" we're talking about? Imagine you're trying to get from your house to a friend's house. You could take a direct route, right? Or, you could detour through a cool little town, stop for a coffee, maybe pick up a snack. Those detours and stops? In the world of chemistry, those are kind of like intermediates.
They're these temporary, often unstable, molecules that pop into existence during a reaction but then quickly get gobbled up to form the final product. Think of them as little helpers, or maybe even accidental tourists, that are part of the process but don't stick around in the end.
Now, what happens when there are no intermediates? It means the reaction is like a super-efficient delivery service. The starting materials (we call these reactants) are zipped straight to becoming the final stuff (the products). No extra stops, no temporary hangers-on. Just a clean, direct transformation.
Why is this even a thing?
You might be thinking, "So what? Does it really matter if there are intermediate steps or not?" Well, as it turns out, it can matter quite a bit! For chemists, understanding the step-by-step process of a reaction is like being a detective. They want to know how things are made, not just that they are made.
Knowing about intermediates can help us understand:
- How fast a reaction will happen: Sometimes, those intermediate steps can be the slowest part of the whole process.
- What other things might be produced: If an intermediate hangs around for a bit, it might decide to do its own thing and create unwanted side products.
- How to control the reaction better: By understanding the pathway, we can sometimes tweak conditions to make the reaction go exactly how we want it to.
So, when a reaction is nice and clean, with no intermediates, it's like a perfectly executed magic trick. Poof! The reactants are gone, and the products have magically appeared. It's elegant, it's efficient, and sometimes, it's just plain cool.
Think of it like this...
Imagine you're making a sandwich. Your reactants are bread, cheese, and ham. Your final product is a delicious sandwich! Now, a reaction with intermediates might involve toasting the bread first, then maybe melting the cheese separately, and then putting it all together. Those are intermediate steps.
But what if you could just grab two slices of bread, slap some cheese and ham between them, and bam! – you have a sandwich? That's a reaction with no intermediates. Super fast, super simple.
Or consider building with LEGOs. You have a pile of bricks (reactants), and you want to build a car (product). A reaction with intermediates might involve building the chassis first, then the wheels, then the body, and finally attaching them. But what if you had a special LEGO kit where you just snapped together three pre-made sections, and there was your car? That's the direct, no-intermediate route!

So, when faced with the question: "When the reaction produces no intermediates, which of the following..."
This kind of question is usually asking you to identify a characteristic or a consequence of such a direct reaction. It's like asking, "If the sandwich magically appeared with no toasting or melting, what can you say about the process?"
Here are some things that are often true when there are no intermediates:
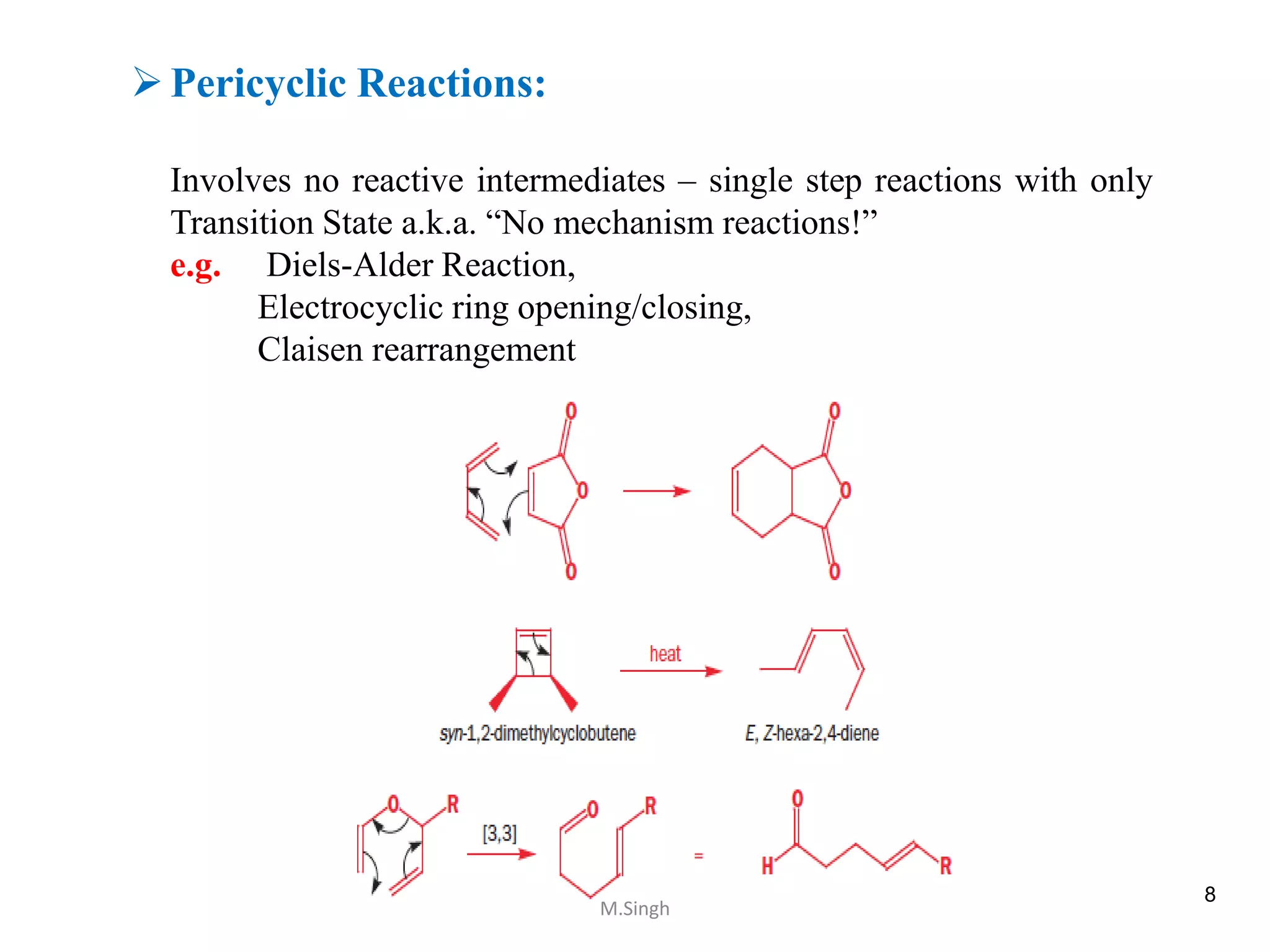
- The reaction is often called a "concerted" reaction. This just means all the bonds are breaking and forming at the same time, in one smooth motion. It's like a perfectly synchronized dance.
- The reaction mechanism is simpler. With no pit stops, the pathway is straightforward.
- It might be harder to control or steer the reaction towards specific outcomes. If there are no distinct intermediate stages, it can be trickier to interrupt or modify the process mid-way.
- The energy profile is different. Instead of having multiple humps (representing energy barriers to get over for each intermediate step), there's usually just one big hump to get over from reactants to products.
In essence, a reaction with no intermediates is a sign of a particularly direct and efficient chemical transformation. It's the chemical equivalent of taking the express train instead of the local one. It gets you there faster, with less fuss.
Why should we care about this simplicity?
Because in science, especially in making new materials or medicines, efficiency is king! If we can design reactions that are direct and produce exactly what we want without any messy side steps, we save time, energy, and resources. It's like finding a shortcut that actually works and doesn't lead you to a dead end.
Understanding when reactions happen in this direct way helps us build better chemical processes. It's fundamental to designing new catalysts that speed things up, or finding ways to make less waste. It’s all about that elegant simplicity.
So, next time you hear about a reaction with no intermediates, give it a little nod. It's a testament to the elegant and often surprisingly direct pathways that chemistry can take. It's a reminder that sometimes, the most complex transformations can happen in the simplest of ways. Pretty neat, huh?
