When 2-methyl-2-butanol Undergoes Dehydration In Acid One Product Is
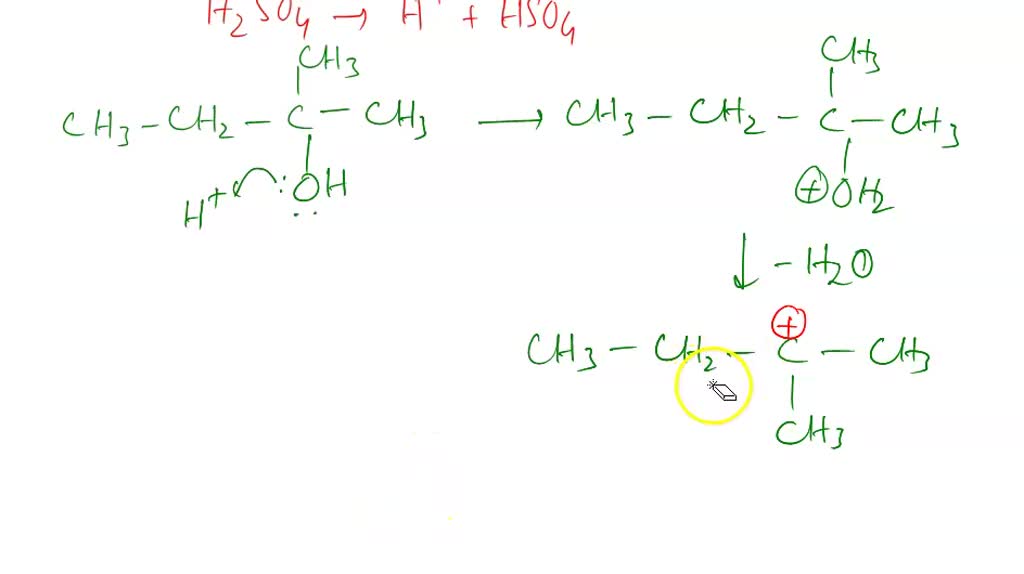
So, you're at a party. Maybe it's a fancy chemistry lab party, or maybe it's just a Tuesday night. You've got this molecule, right? This little guy named 2-methyl-2-butanol. It's a bit of a mouthful, I know. Let's call it "Misty" for short. Misty's just hanging out, minding its own business.
Now, Misty isn't exactly the life of the party. It's kind of a stable molecule. But sometimes, things get interesting when you add a bit of acid to the mix. It's like that one friend who always has a story to tell after a couple of drinks. Suddenly, Misty decides it's time for a makeover.
The acid, bless its bubbly heart, acts like a very enthusiastic hairdresser. It starts to, shall we say, encourage Misty to shed a part of itself. It's a bit dramatic, really. Misty loses a little something, like a pet hair shedding its winter coat.
And what's left behind? Well, that's where the fun begins. Misty, after its little spa treatment, transforms into something new. It’s a bit like a caterpillar turning into a butterfly, but with more… fumes.
The acid is really just saying, "Come on, Misty, let's liven things up!" It pushes Misty to become more unsaturated. You know, like a politician pushing for more buzz. Misty sheds a water molecule. Poof! Gone.
And when Misty loses that water molecule, it opens up the possibility for a little double bond action. Think of it as Misty getting a new, sleeker haircut. It's a bit more edgy now. Less of that clunky single bond thing.
Now, here's the juicy part, and it's a bit of an unpopular opinion I'm about to drop. While Misty can transform into a few different things, one product tends to steal the show. It’s the one that’s just… there. The one that feels like the most obvious outcome, even if the textbooks make it sound like a complex mystery.
This main player, this star of the show, is called 2-methylpropene. Yeah, another long name. Let’s just call it "Mack." Mack is the one you’ll see the most of. It’s like at a wedding, there’s usually one couple that’s just dancing the whole night. Mack is that couple.
Why Mack? Well, it’s all about stability, darling. The universe, much like your Aunt Carol at Thanksgiving, likes things to be as comfortable and settled as possible. And Mack? Mack is feeling pretty darn comfortable.
Think of it this way: Misty has a little arm, and that arm has a hydroxyl group on it. The acid comes along and tickles that hydroxyl group. It makes it a good leaving group, like a guest who’s had too much punch and is ready to go home.
Then, another part of Misty, a neighboring hydrogen atom, gets a little nudge from the acid. It’s like a friendly shove towards the door. And whoosh, the water molecule is born and floats away.
Now, Misty is missing a few things. It has a vacancy, a little hole where the hydroxyl group used to be. And another spot where that hydrogen neighbor took off. This is where the double bond can form.
And Mack, our 2-methylpropene, is a master of this double bond formation. It’s like it planned this. The structure just works out perfectly for it. It’s the molecule that says, "Yep, this is the most sensible place for this new double bond to be."
There are other possibilities, of course. The double bond could theoretically form in a slightly different spot. It’s like saying, "Well, I could go to the karaoke bar, or I could just sit here and judge everyone else."
But Mack is the karaoke king. It’s the most popular choice, the most frequent flyer. It's the one that shows up in the biggest numbers. It’s not that the other options are wrong, per se. They’re just… less likely. Less enthusiastic about forming.
It's kind of like choosing pizza for dinner. You could make sushi, but let's be honest, pizza is usually the go-to. It's reliable. It’s what most people end up with.
So, when 2-methyl-2-butanol, our Misty, gets its acid-induced makeover, and sheds a water molecule, the primary product you’ll find chilling is our friend, 2-methylpropene, or Mack. It’s the star of the dehydration show. The one that makes the biggest impression.
It’s not always the most exciting explanation, I’ll grant you. Sometimes, the simplest answer is the one that’s staring you right in the face. No need for a ten-page report on potential outcomes. Just one big, happy, double-bonded molecule.
And honestly? I kind of appreciate that. In a world full of complicated choices and ambiguous outcomes, it's nice to have a molecule that’s just like, "Yep, this is it. This is the product." No fuss, no muss. Just 2-methylpropene, making its grand entrance.
So next time you hear about 2-methyl-2-butanol dehydration in acid, just picture Misty getting a glow-up and Mack strutting onto the scene. It’s a simple transformation, really. A molecular Cinderella story, with acid as the fairy godmother and Mack as the dashing prince.
And if you ask me, that’s a pretty neat trick. A molecule walks into an acid bath and comes out as something entirely new and, dare I say, cooler. It’s a lesson for us all, really. Sometimes, a little bit of pressure and a good splash of something acidic can lead to wonderful, even if slightly predictable, transformations.
The universe just likes things to be efficient, you know? And Mack is the epitome of molecular efficiency in this particular reaction. It’s the path of least resistance, the most stable option. The molecule that says, "Why make things complicated when you can be this awesome?"
So, there you have it. The simple, entertaining, and dare I say, obvious answer to what one product you get when 2-methyl-2-butanol undergoes dehydration in acid. It's Mack. Always Mack. My unpopular opinion is that it's the most satisfying outcome because it's the one that just makes sense.
It's like choosing the vanilla ice cream. Sure, you could get the obscure exotic flavor, but vanilla is a classic for a reason. It's reliable. It's good. It's 2-methylpropene.
And who doesn't love a bit of predictability in their chemical reactions? Especially when it comes with a nice, juicy double bond. It’s the little victories that count, folks. The molecular equivalent of finding a parking spot right in front of the store.
So, let's raise a (metaphorical) beaker to 2-methylpropene. The unsung hero of 2-methyl-2-butanol dehydration. The product that, in my humble opinion, is just… the one. The most probable, the most stable, and arguably, the most fun.
