What Was The Purpose Of Miller And Urey's Experiment Apex

Okay, picture this. Way back when, before we had smartphones to distract us or even reliable Wi-Fi, scientists were kinda obsessed with a big question: How did life even start on Earth? Like, where did all these squishy, breathing, opinion-having things come from? It’s a question that’s probably popped into your head at least once, maybe while you were stuck in traffic or staring at a particularly uninspired blob of amoeba in a nature documentary. You know, those moments of existential dread that are surprisingly common.
So, these two brainy dudes, Stanley Miller and Harold Urey, decided they were going to have a go at solving this cosmic puzzle. They were basically like, "Let's recreate what we think early Earth was like, and see if we can cook up some life in a test tube." Sounds ambitious, right? It’s like trying to bake a cake without a recipe, and the ingredients are… well, really weird. We’re talking about a primordial soup that was probably less "bouillabaisse" and more "weird swamp water."
Their big idea was to mimic the conditions they imagined existed on Earth billions of years ago. This was the era before fancy cafes and Netflix, a time when the atmosphere was probably a lot more… exciting. We’re talking about gases like methane, ammonia, and hydrogen. Not exactly the air we’re breathing today, which is mostly nitrogen and oxygen. Imagine trying to breathe that stuff! You’d be coughing up a lung before you could even say "good morning." And then there was all that volcanic activity. Lots and lots of heat and lightning. It was basically the ultimate, most intense weather system imaginable. Earth was having a bit of a tantrum, you could say.
So, Miller and Urey set up their experiment. They basically had this contraption that was like a miniature, contained version of early Earth. They had a flask with water to represent the oceans, which they boiled to create "water vapor." Then they sent this vapor through tubes filled with those early-Earth gases. And here’s where it gets spicy: they zapped it all with sparks. Lots of sparks. Like a tiny, controlled lightning storm happening inside a glass box. It was their way of simulating the intense energy that was zipping around on the young planet. Think of it as a prehistoric rave, but with more chemical reactions and less glow sticks.
The whole point was to see if these simple inorganic molecules, under these extreme conditions, could spontaneously form something more complex. Specifically, they were hoping to create amino acids. Now, amino acids might sound like something out of a chemistry textbook, and let’s be honest, they are. But they’re also super important. They are the fundamental building blocks of proteins, and proteins are, well, pretty much everything that makes us, us. They’re in our muscles, our hair, our fingernails, and even the enzymes that help us digest that questionable late-night snack. So, if you can make amino acids, you’re already a big step closer to life.
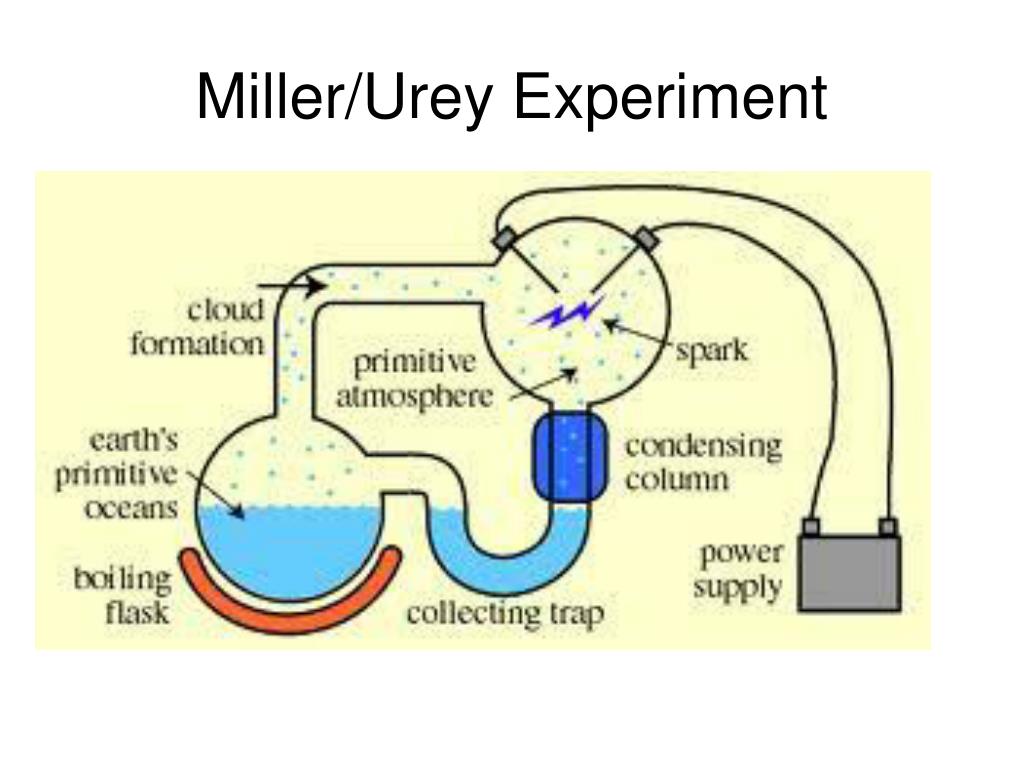
And guess what? They did it! After a week of this simulated primordial soup and lightning show, they found that their water had turned a yucky, brown color. Not exactly appetizing, but to scientists, it was probably like finding a hidden pot of gold. Inside that brown goo, they discovered several different amino acids. Yep, they had managed to create some of the essential ingredients for life from scratch, using just inorganic matter and a whole lot of energy. It was a pretty big deal. It suggested that life’s origins might not have been some miraculous, unfathomable event, but rather a series of natural chemical reactions.
Now, I know what you’re thinking. "But did they make a tiny, fully formed frog?" No, no, not quite. This experiment didn’t suddenly sprout a fully functioning organism. It was more like they managed to bake a single, perfect chocolate chip in a giant kitchen. They proved that the ingredients for life could be formed naturally. It was a crucial piece of the puzzle, showing that the leap from non-living stuff to the building blocks of life wasn’t as impossible as some people might have thought.

Some folks have their reservations, of course. Science is all about questioning, and that’s a good thing. They debate the exact composition of the early Earth’s atmosphere, the role of volcanic vents, and whether it all happened underwater or in some steaming, muddy puddle. And you know what? That’s totally fair. We’re talking about events that happened eons ago, before anyone was around to take notes. It’s like trying to remember what you had for dinner three Tuesdays ago. Pretty fuzzy, right?
But for me, and perhaps for a lot of you out there who just want a good story, the Miller-Urey experiment is a beautiful illustration of our planet’s incredible potential. It’s a reminder that even from the most basic elements, something as complex and wonderful as life can emerge. It’s the ultimate underdog story, where simple ingredients, under just the right conditions, can lead to extraordinary things. So, next time you’re looking at a particularly humble-looking puddle, just remember: it might be the ancestor of something amazing. And that, my friends, is kind of cool, even if it’s not exactly the exciting, life-creating lightning bolt we might have imagined.
