What Structural Problem Prevents Adenine From Pairing With Guanine
Hey there, fellow science enthusiast! Grab your coffee, settle in, because we're about to dive into a little DNA mystery. You know, those tiny building blocks of life that make us, well, us? It’s pretty wild stuff, right?
We’re talking about those famous bases, the As, Ts, Cs, and Gs. Adenine (A), Thymine (T), Cytosine (C), and Guanine (G). They’re like the alphabet of our genetic code. And they have this super important job: pairing up. It's like a constant dance, A always finding its partner T, and C always hooking up with G. So neat!
But have you ever stopped to wonder, why? Like, why doesn't Adenine just decide to pair with Guanine sometimes? I mean, they're both purines, right? Big, double-ringed molecules. It feels like they should be able to mingle. What’s the big deal?
Well, buckle up, buttercup, because the answer is all about their shape. It's a bit like trying to fit a square peg in a round hole, but way more scientifically significant. And a whole lot less frustrating, I promise!
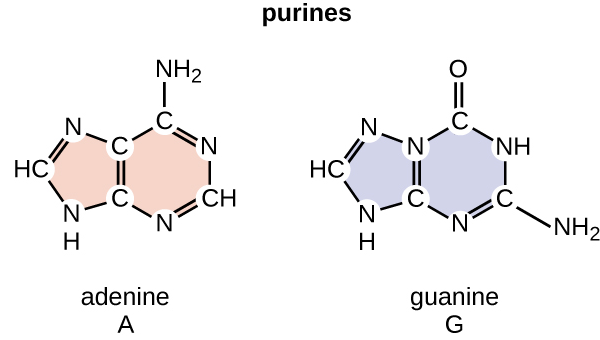
Let's start with our star players, Adenine and Guanine. They're both purines. Think of them as the bigger, more substantial players in the DNA base world. They have two rings fused together. Kind of like a fancy, two-story house. Pretty robust, wouldn't you say?
Now, the other two bases, Thymine and Cytosine, are pyrimidines. These guys are the smaller, more compact ones. Think of them as cozy, one-story cottages. They have just one ring. Simpler, more streamlined.
And here's the kicker: the DNA double helix, that iconic twisted ladder structure, has a very specific width. It's like a meticulously designed tunnel. It needs to be just the right size to hold everything together perfectly.
So, what happens when you try to put two purines together, like Adenine and Guanine? You're essentially trying to cram two two-story houses into a space that's designed for one two-story house and one one-story cottage. It's just… too much. Too bulky. It wouldn't fit neatly. The helix would bulge out like crazy, like you tried to stuff too many clothes into a suitcase. It would mess up the whole structure. Not good for our precious DNA!
Imagine trying to build a LEGO castle with only the biggest bricks. It would be unstable, wobbly, and probably fall over at the slightest breeze. Our DNA needs a bit more finesse, a bit more variation in size, to be strong and stable.
This is where the hydrogen bonds come into play. These are the "glue" that holds the two strands of DNA together. They’re weak individually, but in great numbers, they’re super effective. A and T pair up with two hydrogen bonds. C and G pair up with three. More bonds mean a stronger connection!
But it's not just about the number of bonds. It's also about the placement of the atoms within those molecules. You see, these bases have these little protrusions and indentations, like tiny puzzle pieces. For proper pairing, these shapes need to align just so.
Think of it like trying to high-five someone. You both have to extend your hands in the right way for it to work. If one person turns their hand sideways, or tries to high-five with their elbow, it's just… awkward. And it doesn't create that satisfying connection.
+Thymine+(T)+Guanine+(G)+Cytosine+(C)+Fig.+16-8.jpg)
Adenine and Guanine are both purines, yes, but their internal atomic arrangements are different. They have these nitrogen and carbon atoms in slightly different spots, and these little atoms are crucial. They dictate how the hydrogen bonds can form, and where they can grab on.
When Adenine tries to pair with Guanine, the atoms just don't line up correctly for those crucial hydrogen bonds to form in the right positions. It's like trying to force a key into the wrong lock. It might almost fit, but it won't turn. It won't engage properly.
And let's be clear, this isn't just some arbitrary rule. Nature is all about efficiency and stability. If A and G could just willy-nilly pair up, it would introduce all sorts of errors into the DNA. Imagine if your genetic instruction manual had pages randomly stuck together, or misaligned. Chaos!
The specificity of A-T and C-G pairing ensures that when DNA replicates (makes copies of itself), the information is passed on accurately. It's like having a perfect, error-free photocopier. Every time. Crucial for life, wouldn't you say?
The shape difference between purines and pyrimidines is the primary reason. It's like a size restriction. A purine (big) must pair with a pyrimidine (small). This keeps the width of the DNA helix consistent. Think of a perfectly balanced scale. One side is heavy, the other is light, but they balance each other out.

If you had two purines (big + big), the DNA strand would be too wide. If you had two pyrimidines (small + small), the DNA strand would be too narrow. Both scenarios would be unstable and wouldn't allow for the proper functioning of DNA. It's a Goldilocks situation – not too wide, not too narrow, but just right.
But it goes a little deeper than just the purine-pyrimidine size difference. Even within the purine-purine pairing idea, Adenine and Guanine have slightly different ways they could interact, but those interactions aren't stable or functional for DNA. It’s like two people who could talk, but their communication styles are so incompatible that it just leads to misunderstandings.
The specific arrangement of atoms on Adenine allows it to form two hydrogen bonds with Thymine. It’s a perfect fit, a harmonious handshake. Guanine, on the other hand, has an extra nitrogen atom and a different arrangement that allows it to form three hydrogen bonds with Cytosine. Again, a perfect fit, a more robust embrace!
When Adenine tries to pair with Guanine, the geometry just doesn't allow for these stable hydrogen bonds to form in the correct orientation. There are atoms in the way, or spaces where bonds should be, but aren't. It’s like trying to put on a glove with the wrong hand. It’s just not going to happen smoothly.
So, to sum it up in our casual chat: Adenine is a purine (big), Guanine is a purine (big). DNA needs a purine and a pyrimidine (small) to maintain its consistent width. Think of it as a fundamental rule of construction for our genetic blueprint. Two big guys just don't fit where a big guy and a small guy are supposed to go.
+Guanine+(G)+Purines+Figure+10.2B_2.jpg)
And even if we ignore the size for a second (which we really shouldn't, it's super important!), the way the atoms are arranged on Adenine and Guanine just don't create the right scaffolding for stable hydrogen bonds. It’s like they’re speaking different languages when it comes to forming those crucial connections. Awkward!
So, next time you think about DNA, remember this little detail. It’s not just random pairing. It’s a testament to the elegant and precise structure of life. Adenine and Guanine are meant to pair with their respective partners, Thymine and Cytosine, and there’s a very good structural reason for it. It’s all about keeping that double helix just right, strong, and ready to pass on life’s most important messages.
Isn't science just the coolest? This intricate dance of molecules, all dictated by their shape and how they fit together. Makes you appreciate the complexity and beauty of even the smallest parts of us, doesn't it?
So, there you have it. The structural problem that keeps Adenine and Guanine from getting too friendly. It’s a tale of size, shape, and the perfect arrangement of atoms. A tiny detail that has massive implications for life itself. Pretty mind-blowing, if you ask me!
And remember, this is just a peek behind the curtain. There's so much more to explore! So keep that curiosity alive, keep asking those "why" questions, and who knows what other amazing discoveries you'll make. Maybe you'll be the one to uncover the next big secret!
