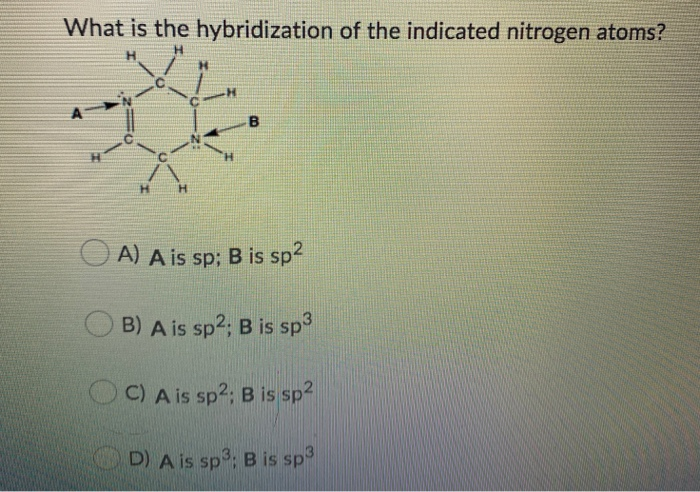
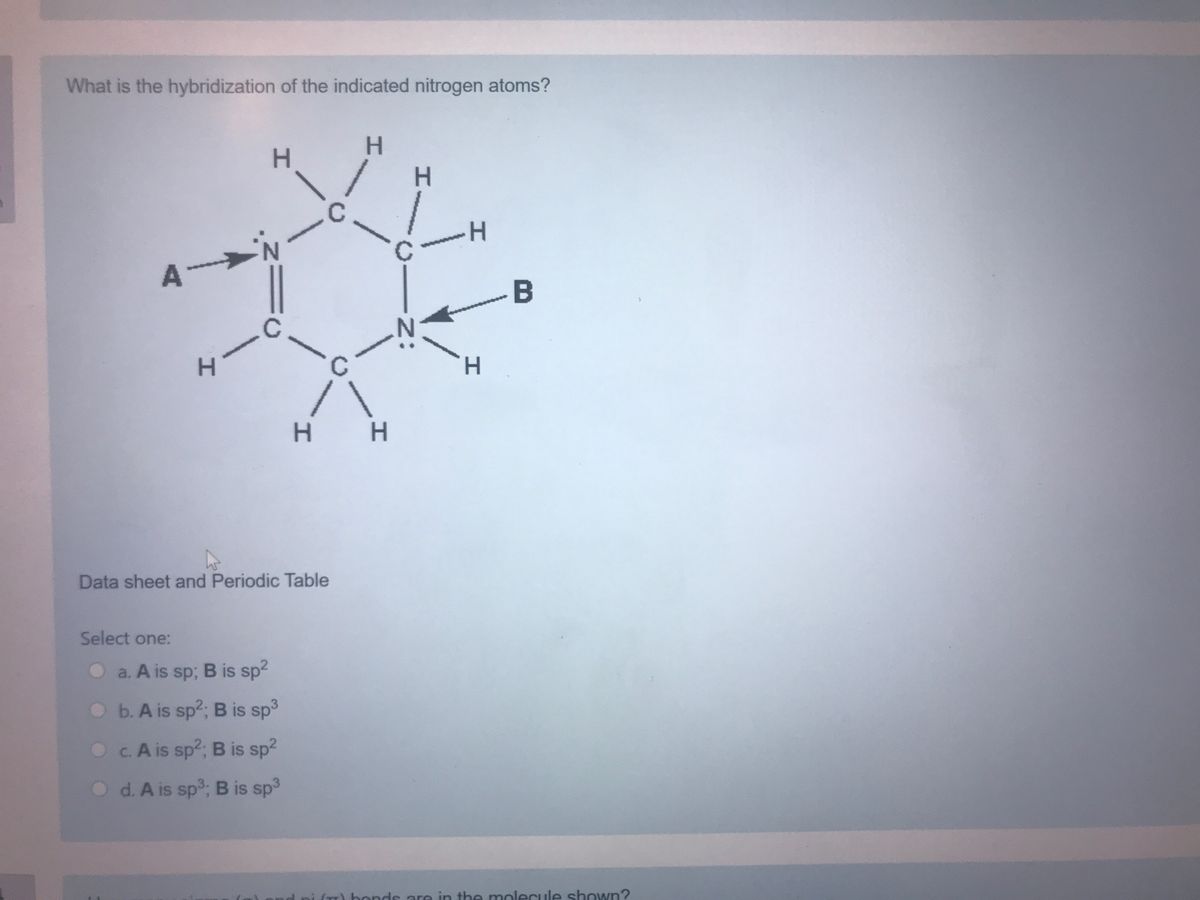
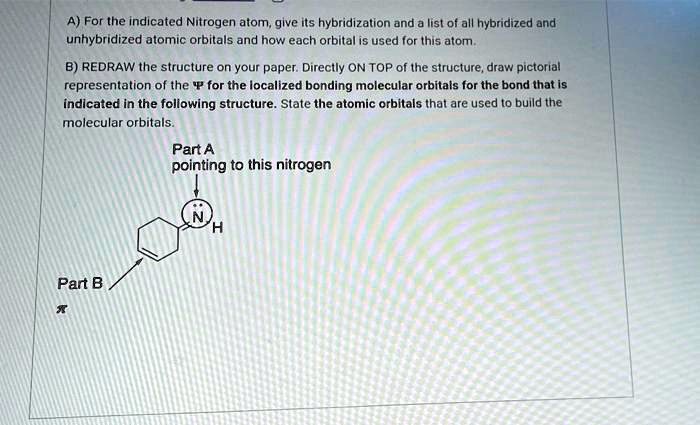
What Is The Hybridization Of The Indicated Nitrogen Atoms

So, you've probably heard whispers about something called "Hybridization." It sounds a bit like a sci-fi movie plot, doesn't it? Like a creature with parts from different animals, or a car that runs on both gas and electricity. Well, in the world of chemistry, it's a bit like that, but for tiny, invisible building blocks called atoms.
Specifically, we're going to chat about the hybridization of nitrogen atoms. Now, nitrogen is super important. It's in the air we breathe, it's in our DNA, and it's in tons of the cool molecules that make up everything around us. It's basically a rockstar element!
Think of an atom like a little dancer. It has different rooms, or "orbitals," where its electrons hang out. These orbitals have different shapes, like spheres and dumbbells. When atoms get together to form molecules, these dancers sometimes need to mix and match their dance moves.
This mixing is what we call hybridization. It's like our dancer deciding to combine their fancy spin with a graceful leap to create a whole new, awesome move. This new move is more stable and better suited for bonding with other dancers.
Now, about our nitrogen friend. Nitrogen usually has a certain number of electrons in its outer shell. When it decides to get cozy with other atoms, like carbon or hydrogen, it needs to prepare for some serious bonding. This preparation involves its electron orbitals getting a makeover.
Imagine nitrogen has a few different dance floors. Normally, these dance floors are separate and have their own distinct styles. But when nitrogen is about to bond, it's like it's saying, "Okay, let's throw all these dance floors together and create a super dance floor!"
This super dance floor isn't just one shape; it's a blend of the original shapes. This blending is the heart of hybridization. It creates new orbitals that are perfect for forming strong chemical bonds. It's like creating custom-made dance shoes that fit just right for a spectacular performance.
For nitrogen, there are a few main ways it can hybridize. It's like there are a few different "levels" of makeover it can get. The most common ones are called sp3 hybridization, sp2 hybridization, and sp hybridization. Each of these gives nitrogen different "dance moves" and shapes for its orbitals.

Let's start with sp3 hybridization. This is like nitrogen taking one "s" orbital (which is like a perfect sphere) and three "p" orbitals (which are like dumbbells) and mixing them all up. It creates four new, identical hybrid orbitals. Think of it as making four identical dance rooms, all ready for action.
These sp3 hybrid orbitals are arranged in a very specific way, kind of like a tetrahedron. This shape is super stable and allows nitrogen to form four single bonds. It’s like having four perfectly positioned arms ready to hug other atoms.
An example of sp3 hybridized nitrogen is found in molecules like ammonia (NH3). Here, the nitrogen atom is bonded to three hydrogen atoms, and it also has a "lone pair" of electrons. This lone pair is like a secret handshake or a little extra flair the nitrogen has.
This neat arrangement in sp3 hybridization is why molecules like ammonia have their specific shapes and properties. It's all about how those electrons are organized, thanks to the hybridization. It’s like a beautifully choreographed dance routine.
Next up is sp2 hybridization. This is a slightly different mix. Here, nitrogen takes one "s" orbital and only two "p" orbitals. It mixes these to create three new, identical sp2 hybrid orbitals. What's left over is one "p" orbital that doesn't get involved in this particular mixing party.
These three sp2 hybrid orbitals are arranged in a flat, triangular shape. They are perfect for forming three bonds. The leftover "p" orbital is like a special performer that can do something extra, like form a double bond.
Molecules with sp2 hybridized nitrogen often have double bonds. A classic example is imines or molecules like nitrate ion (NO3-). In these cases, the nitrogen is involved in a double bond with another atom, which is a stronger connection than a single bond. It’s like a handshake that’s extra firm.
The geometry of sp2 hybridization is planar, meaning it's flat like a pancake. This arrangement is crucial for the reactivity and structure of many organic molecules. It’s like a perfectly laid-out stage for a chemical play.
Finally, we have sp hybridization. This is the most "mixed-up" scenario. Nitrogen takes one "s" orbital and just one "p" orbital. These two mix to form two new, identical sp hybrid orbitals. This leaves two "p" orbitals that are not hybridized and are free to do their own thing.
These two sp hybrid orbitals are arranged in a straight line, like a perfectly aligned row of dancers. They are used to form two bonds, often one single bond and one triple bond, or two double bonds. The two leftover "p" orbitals are super important for forming multiple bonds.
You'll find sp hybridized nitrogen in molecules like cyanides or nitriles. These molecules often have triple bonds, which are incredibly strong. It’s like a chemical super-grip.
The linear geometry of sp hybridization is fascinating. It leads to very specific molecular shapes and influences how these molecules interact with each other. It’s like having a perfectly straight runway for chemical reactions to take off.
So, why is this whole hybridization of nitrogen thing so entertaining? It’s like a behind-the-scenes peek at how molecules are built. It's the secret sauce that gives molecules their unique personalities and capabilities.
It's not just abstract theory; it directly impacts the world around us. The way drugs work, the way proteins fold, the way plants grow – all of this is influenced by the precise way atoms like nitrogen bond, and hybridization is key to that. It’s like the invisible engineering that holds our universe together.
What makes it special is the elegance of it all. How simple atomic orbitals, when mixed in specific ways, create the perfect tools for building complex and stable molecules. It’s a beautiful example of nature’s efficiency and ingenuity.
It's like discovering that your favorite building blocks can transform into different shapes depending on how you connect them. This adaptability is what makes nitrogen such a versatile player in the chemistry game. It can be shy and form single bonds, or it can get really enthusiastic and form double or triple bonds.
Thinking about hybridization can make you see molecules in a whole new light. Instead of just static structures, you start to see them as dynamic entities with specific spatial arrangements and bonding patterns. It's like upgrading from a black-and-white photo to a 3D IMAX movie.
The different types of hybridization (sp3, sp2, sp) offer a kind of "choose your own adventure" for nitrogen's bonding behavior. Each choice leads to a different molecular structure and a different set of properties. It’s like nitrogen has a whole wardrobe of bonding outfits.
This concept helps us understand why some molecules are stable and others are reactive. It explains the geometry of molecules, which is critical for their function. It's the underlying logic of molecular architecture.
So, the next time you hear about hybridization of nitrogen atoms, don't be intimidated. Think of it as the atomic equivalent of a clever costume change that allows nitrogen to perform its essential roles in countless chemical reactions. It's a fascinating glimpse into the molecular world that makes everything tick.
It’s a reminder that even the smallest parts of nature have incredible complexity and beauty. The way these atoms arrange themselves, driven by fundamental principles like hybridization, is truly a marvel. It’s the invisible dance of atoms that creates the visible world.
Hopefully, this little peek into the world of nitrogen's bonding adventures has sparked your curiosity. It's a deep and rewarding subject, and understanding it can open up a whole new appreciation for the chemistry happening all around you, and even inside you!
So next time you’re looking at a chemical structure, remember the hidden work of hybridization making it all possible!
