What Is The Hybridization Of The Carbon Atom In Cs2
Alright, gather 'round, science enthusiasts and the just plain curious! We're about to dive into a tiny, yet utterly fascinating, world: the amazing adventures of carbon atoms! You know, those little guys that are the backbone of everything from your morning coffee to the magnificent redwoods. Today, we're going to tackle a specific, and dare I say, sparkling example: the carbon atom in Cs₂.
Now, you might be thinking, "Cs₂? What in the world is that?" Well, think of it like a very fancy, very exclusive club for cesium atoms. Cesium (Cs) is a real show-off in the alkali metal family, always eager to shed an electron and become positively charged, like a celebrity signing autographs. And when two of these flamboyant cesium atoms get together, they form Cs₂! It's like a double-dose of metallic charm.
But where does our humble carbon atom fit into this picture? This is where things get really interesting, and a little bit like a cosmic surprise party. In the case of Cs₂, the carbon atom isn't just chilling out; it's actually playing a crucial role, acting as the glue, the maestro, the heart of this whole operation. Imagine it as the shy, brilliant artist at a wild party, making everyone else look good.
So, how does this carbon do its magic? It involves something super cool called hybridization. Now, don't let that word scare you! Think of hybridization like this: imagine you have a bunch of Lego bricks of different shapes and sizes. Hybridization is like taking those bricks and, with a bit of atomic-level ingenuity, melting them down and reforming them into brand new, perfectly shaped bricks. These new bricks are even better for building whatever needs to be built.
In the case of our carbon atom in Cs₂, it's like it’s got a few different "talent rooms" it can use for its atomic dance. Normally, carbon has some talents it keeps to itself (those are its pure atomic orbitals) and some it likes to share and mix up (those are its valence electrons). But when it's part of something special like Cs₂, it’s ready to go all out.
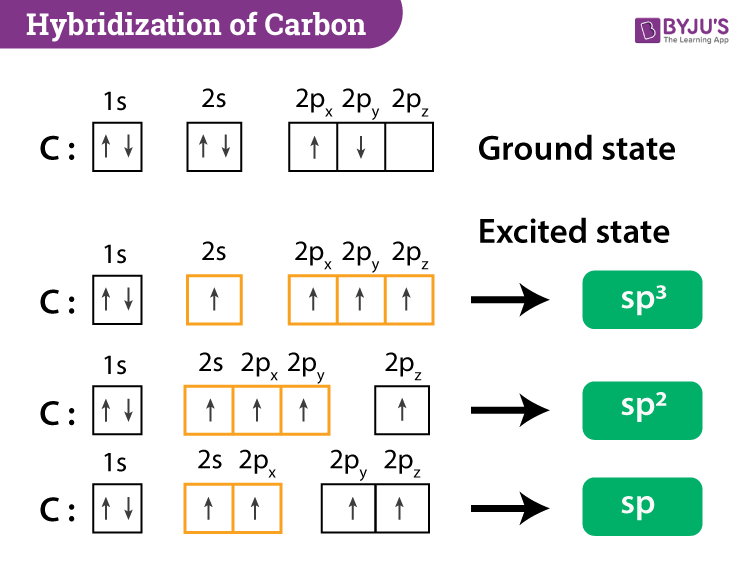
So, the carbon atom in Cs₂ is a prime example of sp hybridization. That might sound like a secret code, but it's actually quite straightforward. It means our carbon friend takes one of its "s" talent rooms and one of its "p" talent rooms and poof! – it smashes them together to create two new, super-powered "sp" talent rooms. These new rooms are identical and are perfectly angled, like two perfectly aimed spotlights.
Why does it do this? Because it's building a magnificent structure! These two new sp hybrid orbitals are absolutely essential for forming the strong, linear connections that carbon needs to do its job in Cs₂. It’s like having two perfectly designed tools for a very specific job, ensuring maximum efficiency and stability. It's the atom equivalent of wearing a perfectly tailored suit.
Now, remember those other "p" talent rooms that carbon had? The ones it didn't use for the sp hybridization? Well, they don't just sit idle! They're like bonus talents, ready to be used for other awesome atomic interactions. In Cs₂, these leftover "p" orbitals are available to form those very special, sideways connections that we call pi (π) bonds.

Think of a regular bond, like a firm handshake, as a sigma (σ) bond. It’s direct and strong. Now, a pi bond is like a sneaky, side-hug that happens above and below the main handshake. These pi bonds are super important for creating rigidity and allowing for those cool, delocalized electron effects that make molecules behave in fascinating ways. It's like adding a splash of glitter and a confetti cannon to the handshake!
So, in Cs₂, our carbon atom, with its sp hybridization, forms a strong sigma bond with whatever it's connected to (in this case, likely a cesium atom, though the exact structure of Cs₂ itself is a bit more complex and involves polyatomic anions). And then, those extra "p" orbitals get to work, forming those delightful pi bonds. It's a masterful display of atomic engineering, all happening at speeds you can't even imagine!
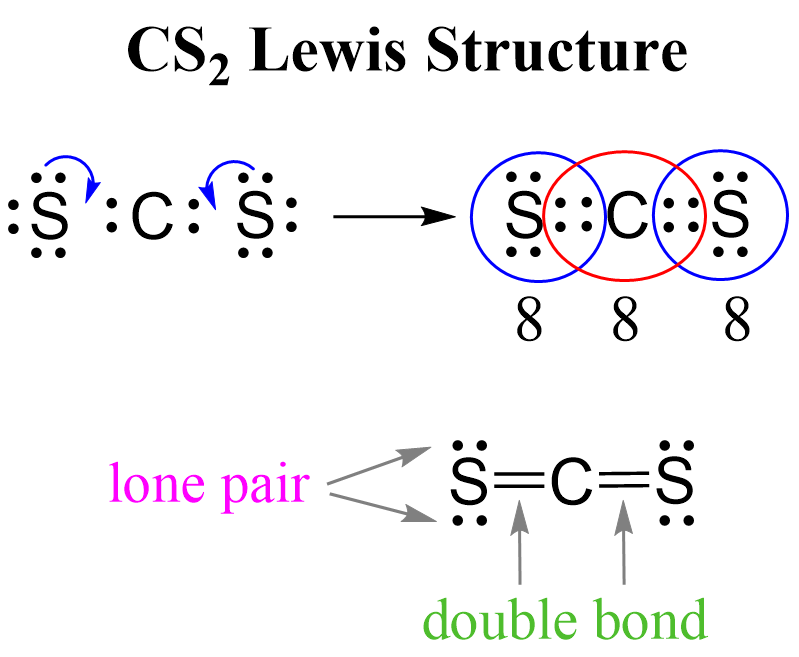
The result is a molecule that is not only stable but also possesses unique electronic properties. The cesium atoms, being so eager to give away their electrons, are perfectly balanced by the carbon atom's versatile hybridization. It’s a beautiful synergy, a cosmic dance of elements where each atom plays its part perfectly.
It’s like a perfectly choreographed ballet. The cesium atoms are the flashy prima ballerinas, and the carbon atom is the sophisticated choreographer, using its sp hybridized orbitals and leftover p orbitals to create a stunning and stable performance. The sigma bonds are the strong foundational steps, and the pi bonds are the elegant leaps and twirls that make the whole routine captivating.
Isn't that just the coolest? The fact that an atom can rearrange its internal "talent rooms" to perfectly suit the needs of the molecule it's in is mind-blowing. And the sp hybridization of carbon in compounds like Cs₂ is a testament to its incredible versatility and its fundamental importance in the universe of chemistry. It's like carbon is the ultimate shape-shifter, always ready to adapt and contribute to something amazing.
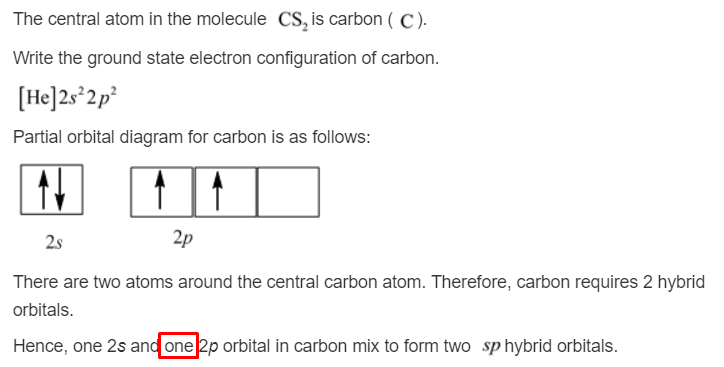
So next time you hear about hybridization, or see a formula like Cs₂, remember this little atomic party. Remember the carbon atom, flexing its sp hybridization muscles, using its pure p orbitals for those fancy pi bonds, and generally being the absolute rockstar of the molecular world. It’s a tiny detail, but it’s the kind of detail that makes the universe sing with wonder!
It just goes to show that even the smallest building blocks of the universe are capable of incredible feats of adaptation and bonding. The carbon atom, with its magical ability to hybridize, is truly one of nature's most ingenious creations. And seeing it in action within the context of something like Cs₂ is like getting a VIP backstage pass to the greatest show on Earth – the show of chemistry!
So there you have it! A little peek into the extraordinary world of carbon and its sp hybridization in the intriguing compound Cs₂. Keep your eyes peeled, because the universe of chemistry is full of these delightful surprises, just waiting to be discovered. And who knows, maybe you'll even find yourself humming a little atomic tune of your own! The adventure never truly ends when you start exploring the magic of atoms!
