What Is The Equilibrium Constant Of A Reaction Apex

Hey there, science curious folks! Ever wondered about the secret life of chemical reactions? You know, the ones happening all around us, from baking a cake to the very air we breathe? Well, buckle up, because we're diving into something super interesting that helps us understand these reactions a bit better: the equilibrium constant. Don't let the fancy name scare you – it's actually a pretty neat concept!
So, what exactly is this equilibrium constant? Imagine you're at a bustling party. People are chatting, mingling, and some are even heading for the exit while others are just arriving. It’s a constant flow, right? But after a while, even though there's lots of movement, the total number of people inside the party might stay pretty much the same. That's kind of like chemical equilibrium. It's that sweet spot where a reversible reaction – one that can go both forward and backward – reaches a state where the rate of the forward reaction (reactants turning into products) is equal to the rate of the reverse reaction (products turning back into reactants). It's not that the reaction stops, oh no! It's just that the dance between forming and breaking bonds has found its rhythm.
Now, the equilibrium constant (often shortened to just 'K') is like a little scorekeeper for this party. It tells us something crucial about where this equilibrium lies. Does the party lean more towards having lots of people arriving and staying, or are people mostly heading out? In chemistry terms, does the reaction tend to favor making more products, or does it prefer to hang out with more reactants? K is the number that gives us this insight.
Think of it like a seesaw. On one side, you have your reactants (the ingredients), and on the other side, you have your products (what you end up with). A reversible reaction means the seesaw can tilt in both directions. The equilibrium constant is basically telling us how much the seesaw is tilted when it’s perfectly balanced. If K is a really big number, it means the seesaw is heavily tilted towards the products. So, at equilibrium, you'll have way more products than reactants. It's like the party where everyone's having such a blast they don't want to leave!
On the flip side, if K is a tiny, tiny number (much less than 1), it means the seesaw is tilted heavily towards the reactants. So, at equilibrium, you’ll find mostly reactants hanging around, with only a little bit of product formed. This is like a party where most people just pop in for a minute and then head off. The reaction, in this case, doesn't really go very far towards making products.
And if K is somewhere around 1? Well, that's like a perfectly balanced seesaw. At equilibrium, you'll have a good mix of both reactants and products. The party is lively, with arrivals and departures happening at a balanced pace.
So, why is this so cool?
Well, for starters, it's incredibly predictive. Scientists don't have to guess. By knowing the value of K, they can get a really good idea of how a reaction will behave under specific conditions. This is super important for so many things!
Take the Haber-Bosch process, for example. This is the industrial method for making ammonia (NH3) from nitrogen (N2) and hydrogen (H2). Ammonia is a huge deal for us – it’s a key ingredient in fertilizers, which help grow the food that feeds the world! The reaction is:
N2 (g) + 3H2 (g) ⇌ 2NH3 (g)
Now, this reaction has an equilibrium constant. If K were really small, it would mean that at equilibrium, you’d barely get any ammonia. That would be a bummer, right? Luckily, for this reaction, K is reasonably favorable, especially at certain temperatures. But there's a catch! Higher temperatures speed up reactions, which sounds good, but for this specific reaction, higher temperatures actually decrease the equilibrium constant, meaning less ammonia is made at equilibrium. So, chemists have to find a clever balance – using moderately high temperatures to make the reaction happen fast enough, but not so high that they drastically reduce the amount of ammonia they can get at equilibrium. They also play with pressure and catalysts. It's a whole chemical juggling act, and the equilibrium constant is a vital piece of information in figuring out how to make it work efficiently.
Think of it like trying to make the perfect cup of tea. You have your tea leaves (reactants) and your hot water. The tea infusion (product) happens. But you can't just dunk the leaves for a second and expect strong tea, nor can you leave them in forever because it’ll get bitter. There’s an optimal steeping time where you get the best flavor. The equilibrium constant is like knowing that perfect steeping time for a chemical reaction – it tells you when the "flavor" (the product) is just right.
How do we actually get this K number?
It’s not just pulled out of thin air! The equilibrium constant is calculated based on the concentrations (or partial pressures for gases) of the reactants and products once the reaction has reached equilibrium. For a general reversible reaction like:
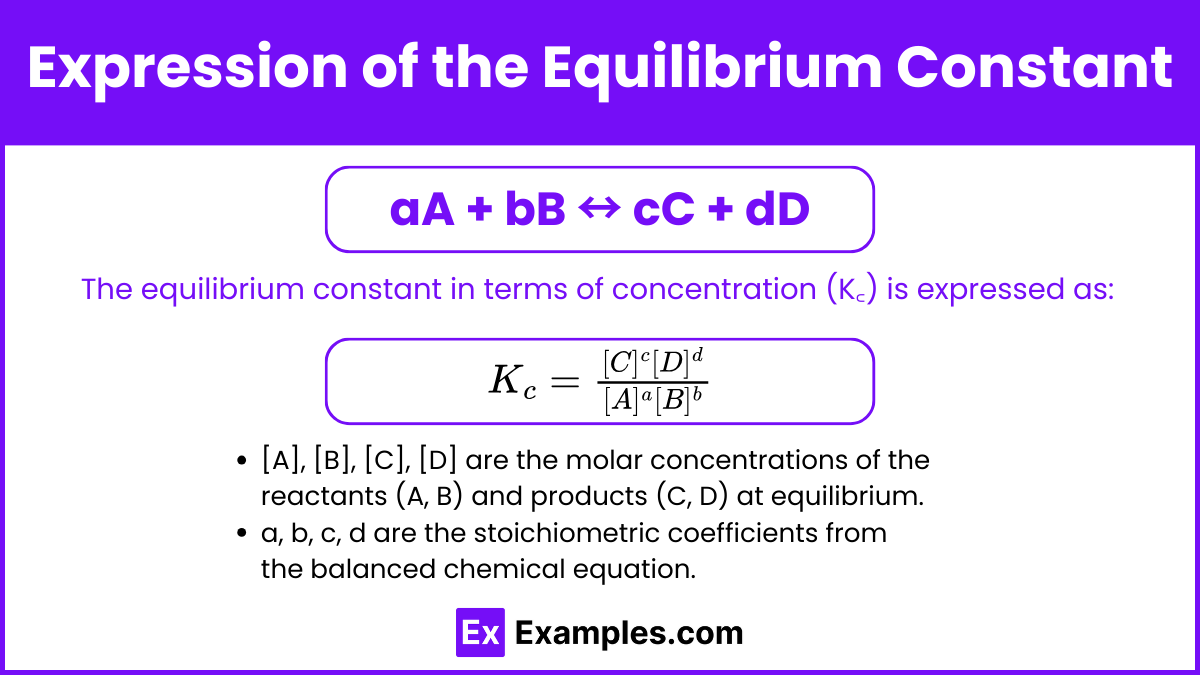
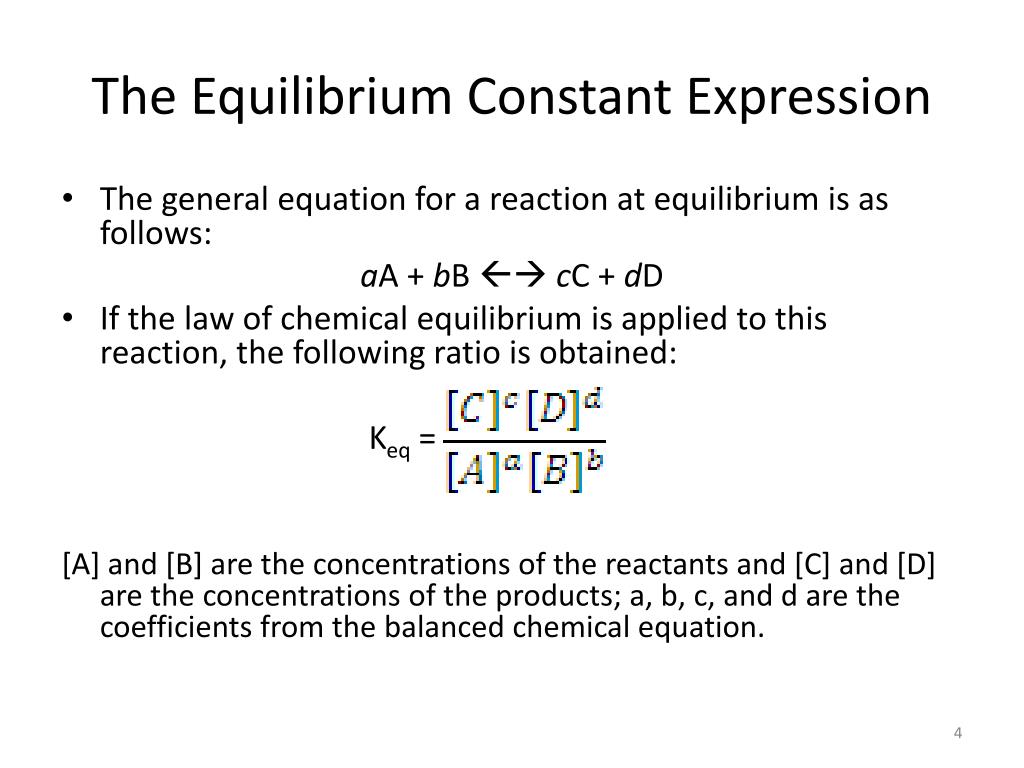
aA + bB ⇌ cC + dD
(where A and B are reactants, C and D are products, and a, b, c, and d are their respective stoichiometric coefficients from the balanced equation), the equilibrium constant (Kc for concentrations, Kp for pressures) is calculated as:
Kc = ([C]^c * [D]^d) / ([A]^a * [B]^b)

See what's happening there? The concentrations of the products (raised to the power of their coefficients) go on top, and the concentrations of the reactants (also raised to their coefficients) go on the bottom. This formula is directly derived from the idea we talked about earlier: a bigger K means more products relative to reactants.
It's a bit like a recipe's rating system. If you're rating a cake recipe, you'd likely give higher points for recipes that consistently produce delicious cakes (lots of product) and lower points for those that don't turn out so well (little product). The equilibrium constant is that rating for chemical reactions.
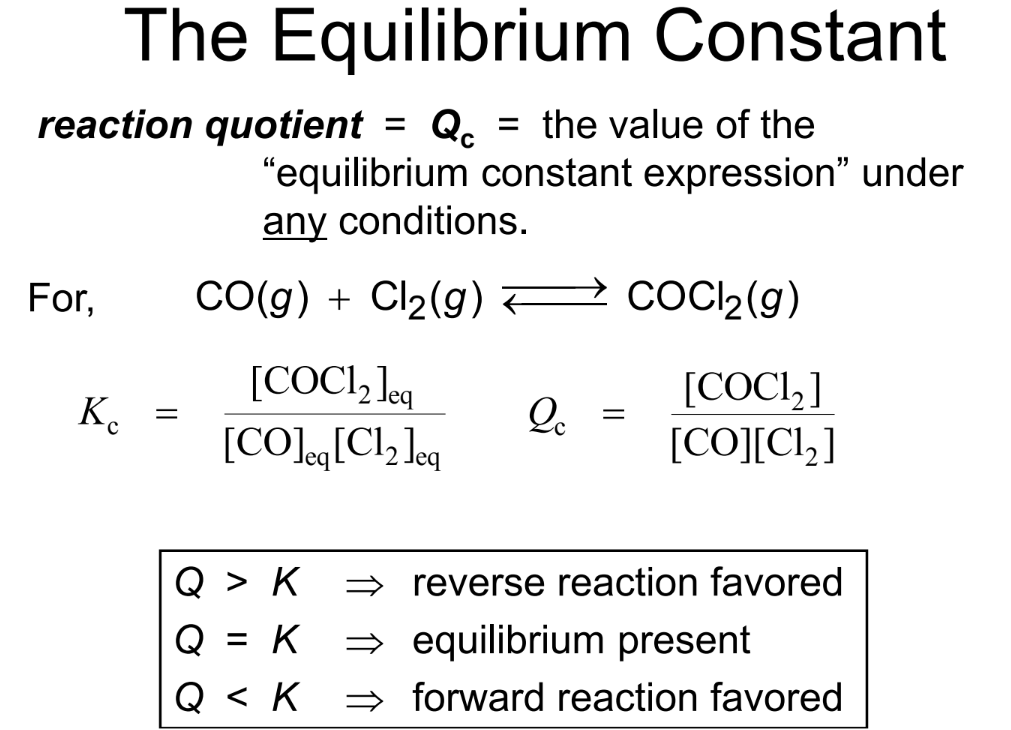
The neat thing is that K is pretty stable for a given reaction at a specific temperature. Change the temperature, and K will likely change. But as long as the temperature stays the same, K is your reliable guide to the reaction's tendency. It doesn't tell you how fast the reaction gets to equilibrium (that's kinetics, a different but related topic!), but it tells you where it's going.
So, next time you hear about a chemical reaction, remember the equilibrium constant. It’s a simple number, but it unlocks a whole lot of understanding about the balance of the chemical world. It’s a fundamental concept that helps us design processes, understand natural phenomena, and even create new materials. Pretty cool for something so seemingly abstract, right?
