What Is The Equilibrium Constant For The Following Reaction

Ever stare at a recipe and wonder why, no matter how perfectly you follow the steps, it sometimes turns out slightly different? Or maybe you've noticed how a game of tug-of-war can reach a point where neither side is gaining ground – a sort of stalemate, right? Well, guess what? The universe is a lot like a giant, cosmic kitchen and a never-ending tug-of-war, and there's a fancy scientific term for that equilibrium point: the equilibrium constant! Sounds a bit intimidating, doesn't it? Like something you’d need a lab coat and safety goggles for. But stick with me, because this little concept is surprisingly fun and actually has a lot to say about… well, everything!
So, what exactly is this "equilibrium constant" we're yapping about? Imagine you're baking your famously delicious chocolate chip cookies. You’ve got your ingredients (reactants), and you bake them up into glorious cookies (products). But here's the twist: with some chemical reactions, it’s not a one-way street. The cookies can actually un-bake themselves back into dough! (Okay, maybe not cookies, but you get the idea). These are called reversible reactions. They're constantly going forward (making stuff) and backward (un-making stuff) at the same time. It’s like a dance, a constant back-and-forth.
Now, at some point in this chemical dance, things settle down. It's not that the reaction stops – oh no, the dance continues! But the speed of the forward dance (making cookies) becomes exactly the same as the speed of the backward dance (un-baking cookies). This, my friends, is chemical equilibrium. It's that perfect moment where the amount of cookies and the amount of dough are staying constant. Not necessarily equal, mind you, just constant. Think of it as the ultimate balance, a state of peaceful coexistence between your reactants and products.
So, Where Does the "Constant" Come In?
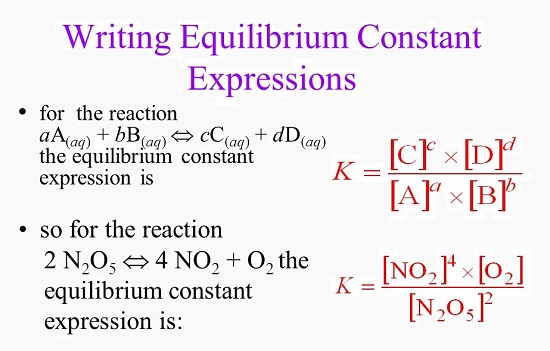
Ah, the equilibrium constant (often shown as a capital 'K' – simple, right?) is basically a number that tells us where this perfect balance point lies. It's like a scorekeeper for the reaction's dance. This number is calculated using the concentrations of your reactants and products when the system has reached that sweet equilibrium. It’s a ratio, a comparison. Don't worry, we're not going to dive into the nitty-gritty math formulas today (unless you really want to – then you can find them with a quick search!). The important thing to grasp is that this 'K' value is a fixed number for a specific reaction at a specific temperature. It doesn't change unless you mess with the temperature (which is like changing the music tempo of our dance).
What does a big 'K' mean? If your equilibrium constant is a large number, it means that at equilibrium, you'll have a whole lot more products than reactants. Your reaction has a strong tendency to go forward. It's like your cookie recipe is so amazing, it practically wants to be cookies! It’s a successful dance party, with way more dancers on the "product" side.
+%2B+3H2(g)+2NH3(g).jpg)
Conversely, if 'K' is a small number, it tells you that at equilibrium, you'll have way more reactants hanging around. The reaction doesn't really like to go forward much. It's like your recipe is a bit shy, and most of the ingredients prefer to stay in their original form. The dance party is a bit of a dud, with most of the dancers on the "reactant" side.
And if 'K' is around 1? Well, that's the sweet spot! It means you'll have roughly equal amounts of reactants and products at equilibrium. A pretty balanced dance, a harmonious coexistence. Neither side is really dominating the floor.
Why is This Even Cool?
You might be thinking, "Okay, that's neat, but how does this make my life more fun?" Well, think about it! This concept applies to so many things. When you brew your morning coffee, there's an equilibrium happening! The coffee grounds are dissolving into the water, and some of the dissolved coffee is reattaching to the grounds. The 'K' value for that process helps determine how strong your coffee will be. A higher 'K' means more coffee goodness in your mug!
Or consider how medicines work in your body. Your body is a complex chemical soup, and many drugs work by reaching an equilibrium with certain molecules. Understanding the equilibrium constant helps scientists design drugs that are effective. It's like tuning an instrument to get the perfect note – we're tuning chemical reactions to get the perfect biological outcome!
Even something as simple as a fizzy drink relies on equilibrium. The carbon dioxide gas is dissolved in the liquid, and there's a balance between gas that's dissolved and gas that's escaped. When you open the can, you disturb that balance, and poof – bubbles galore! It's a little chemical fireworks show driven by equilibrium.

This knowledge empowers you to understand the world around you a little better. It takes the mystery out of certain processes and turns them into fascinating scientific stories. Instead of just using things, you can start to appreciate the intricate chemical ballets happening all around you, all the time. It's like gaining a superpower – the superpower of chemical insight!
The Joy of Understanding
The beauty of the equilibrium constant is its simplicity and its universality. It’s a fundamental principle that governs how reactions behave. It’s a constant reminder that even in apparent stillness, there's often a dynamic, ongoing process. It's about finding balance, about reaching a state of dynamic harmony. And isn't that something we all strive for in our own lives? A little bit of equilibrium, a little bit of balance amidst the chaos.
So, the next time you enjoy a perfectly brewed cup of tea, marvel at a bubbling cauldron of science, or simply ponder the gentle fizz of your favorite soda, remember the unsung hero: the equilibrium constant. It’s a concept that, once you understand it, can unlock a whole new layer of appreciation for the intricate, amazing, and often surprisingly fun world of chemistry. It’s not just numbers on a page; it's the secret language of how things change and settle. Go forth, and let the wonder of chemical equilibrium brighten your day!
