What Is Meant By The Simplest Formula Of A Compound

Hey there, coffee buddy! So, you're curious about this whole "simplest formula of a compound" thing, huh? Like, what's the big deal? Is it just another one of those chemistry terms that sounds way more complicated than it actually is? Well, grab another sip, because we're about to break it down. And I promise, it's not going to be like dissecting a frog in high school science class. Phew!
Think of it like this: imagine you're at a party, and you've got all these amazing ingredients for a super fancy cake. You've got flour, sugar, eggs, butter, chocolate chips – the works! Now, you could list every single one of those ingredients, right? Every single grain of sugar, every speck of flour. But honestly, who has time for that? And who really cares about the exact number of sprinkles on top, unless you're, like, a sprinkle fanatic?
The simplest formula is kind of like saying, "Okay, the main ingredients for this cake are flour, sugar, eggs, and butter. And maybe some chocolate chips for good measure." It gives you the essence of what's in there, without getting bogged down in the nitty-gritty details. It's the gist of the recipe, you know?
In chemistry, a compound is basically a mix of different elements. Like water, H₂O. Super common, right? We all know that. But what if we were talking about something a bit more… complex? Something with a really, really long chemical formula. Like, imagine trying to list every single atom in a giant molecule of DNA. My brain already hurts just thinking about it!
That's where our friend, the simplest formula, swoops in to save the day. It’s also often called the empirical formula. Fancy, right? Sounds like something you’d find in an old dusty encyclopedia. But really, it’s just about simplifying things. Making them manageable. Like when you tell your friend you're going to a "barbecue" instead of listing every single type of sausage and marinade you plan to use.
So, what does it mean to be the simplest formula? It means we’re looking at the smallest whole-number ratio of atoms of each element in a compound. Think of it as the most basic building block, the fundamental ratio. No fractions, no decimals, just good old whole numbers. It’s like saying, "For every two hydrogen atoms, there's one oxygen atom in water." Simple as that.
Let’s take our buddy glucose. You know, that sugar that gives you that energy boost (and sometimes a sugar crash, oops!). Its full chemical formula is C₆H₁₂O₆. That’s six carbons, twelve hydrogens, and six oxygens. Now, if you’re trying to explain glucose to someone who’s never heard of it, saying "six, twelve, six" might be a bit much. It's like giving someone directions to your house and listing every single pothole on the way.

But, if we look at the ratio of carbon, hydrogen, and oxygen in glucose, we see a pattern. We can divide all those numbers by… wait for it… six! Yep, six is the magic number here. So, for every 6 carbons, we have 12 hydrogens (which is 2 times 6), and for every 6 oxygens, we have 6 oxygens. See the pattern? It's a 1:2:1 ratio.
So, the simplest formula for glucose is CH₂O. Bam! Much cleaner, much easier to remember. It’s like the nickname for the compound. Instead of calling your friend "Jonathan Bartholomew Reginald the Third," you just call him "Jon." Much more manageable, wouldn't you agree?
This simplest formula, or empirical formula, tells us the proportion of elements. It doesn't necessarily tell us the actual number of atoms in a molecule. For example, CH₂O is the simplest formula for glucose, but it’s also the simplest formula for formaldehyde. Weird, right? It’s like two different people having the same nickname. It can be a bit confusing at first, I get it. But it's important to remember that the context usually tells you which one you're dealing with.
Think of it this way: if someone says "I'm craving something sweet," you don't automatically assume they want a giant chocolate cake. They might be happy with a piece of fruit, or a small cookie. CH₂O is like the "something sweet" option. It gives you the basic idea of what’s going on without all the extra fluff.
So, why do chemists even bother with this simplest formula stuff? Well, imagine you’re trying to figure out the composition of a new compound you just discovered. You can’t just look at it and know exactly how many atoms of each element are in there. You have to do some experiments, analyze the percentages of each element present. And from those percentages, you can figure out the simplest formula.

It’s like being a detective. You find clues (the percentages of elements), and then you piece them together to get the full picture. The simplest formula is often the first step in identifying a brand new substance. It’s the starting point for figuring out the real formula.
Let’s say you do an experiment and find out that a compound is made up of 40% carbon, 6.7% hydrogen, and 53.3% oxygen. Now, what do you do with those numbers? You don’t just stare at them and hope for the best, right? You convert those percentages into grams, assuming you have, say, 100 grams of the compound. So, 40 grams of carbon, 6.7 grams of hydrogen, and 53.3 grams of oxygen.
Then, you take those grams and convert them into moles. Because in the world of chemistry, we tend to think in moles. It's just a number, a really big number (Avogadro's number, if you want to get all technical). So, you divide the mass of each element by its atomic mass. For carbon, that's about 12 g/mol. For hydrogen, about 1 g/mol. And for oxygen, about 16 g/mol.
After you do those calculations, you’ll get some numbers. They might not be nice, round whole numbers. They might be something like 3.33 moles of carbon, 6.7 moles of hydrogen, and 3.33 moles of oxygen. See those 3.33s? They’re pretty close to each other. And 6.7 is roughly double 3.33. Coincidence? I think not!

So, to get the simplest whole-number ratio, you divide all those mole numbers by the smallest one. In this case, it's 3.33. So, 3.33 divided by 3.33 is 1. 6.7 divided by 3.33 is approximately 2. And 3.33 divided by 3.33 is 1. Voila! You’ve got your 1:2:1 ratio. And that, my friend, brings you back to CH₂O!
Pretty neat, huh? It’s like a puzzle where the pieces are atoms, and the simplest formula is the completed picture, just maybe a simplified version of the full artwork. It’s the skeleton key to understanding the basic composition of a compound.
Now, sometimes, the actual molecular formula is just a multiple of the simplest formula. For example, if you’ve got a compound where the simplest formula is CH₂O, but you know it has more atoms than that. Maybe the molecular formula is C₂H₄O₂. See? That’s just CH₂O multiplied by 2. Or it could be C₆H₁₂O₆ (our friend glucose!), which is CH₂O multiplied by 6.
So, the empirical formula is like the base unit. The building block that you multiply by some integer to get the actual molecular formula. It's the most reduced version. Like when you simplify a fraction, right? 2/4 becomes 1/2. 1/2 is the simplest form.
Why would you need both? Well, the molecular formula tells you the actual number of atoms of each element in a molecule. That's super important for understanding how a molecule behaves. It's like knowing not just that a cake has flour and sugar, but how much flour and how much sugar. That's crucial for baking, right?
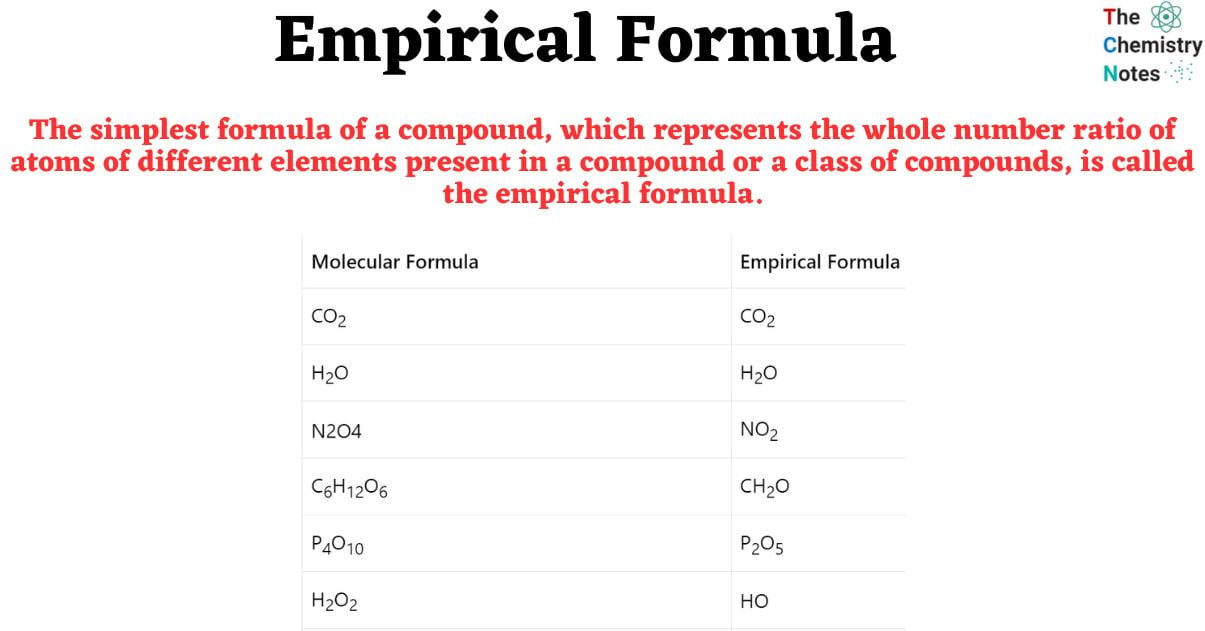
But the simplest formula is still incredibly useful. It's easier to work with, easier to remember, and as we saw, it's what you often get directly from experimental data. It’s the first clue, the starting point for figuring out the more complex stuff.
Think about it like this: if you're designing a house, you might start with a basic floor plan. That floor plan is like the simplest formula – it shows the general layout and the relationships between rooms. Then, you add all the details: the paint colors, the furniture, the fancy light fixtures. That’s like the molecular formula, the complete picture.
So, to recap, the simplest formula of a compound is the most reduced ratio of atoms of each element in that compound. It’s represented by whole numbers and gives you the fundamental building blocks. It’s the empirical formula, the essence of the compound’s composition.
It’s not always the real molecular formula, but it’s a crucial step in determining it. It's the foundation upon which the more complex structure is built. And honestly, in a world that can feel pretty complicated, sometimes a little bit of simplification is exactly what we need, right? It’s like finding a calm little oasis in the middle of a bustling city.
So next time you hear about the simplest formula, don’t get intimidated. Just think of it as the chemistry equivalent of a really good summary. It gets straight to the point, tells you what you need to know, and makes the whole subject a little bit easier to digest. And who doesn't love a good summary, especially when coffee is involved? Cheers!
