What Happens To Energy During The Formation Of A Solution
Hey there, science adventurer! Ever just dump some sugar into your coffee and think, "Wow, that just disappeared!"? Well, get ready for a little peek behind the magic curtain. We're gonna talk about what happens to all that oomph when stuff dissolves. It's way more interesting than you think, and honestly, a little bit wild.
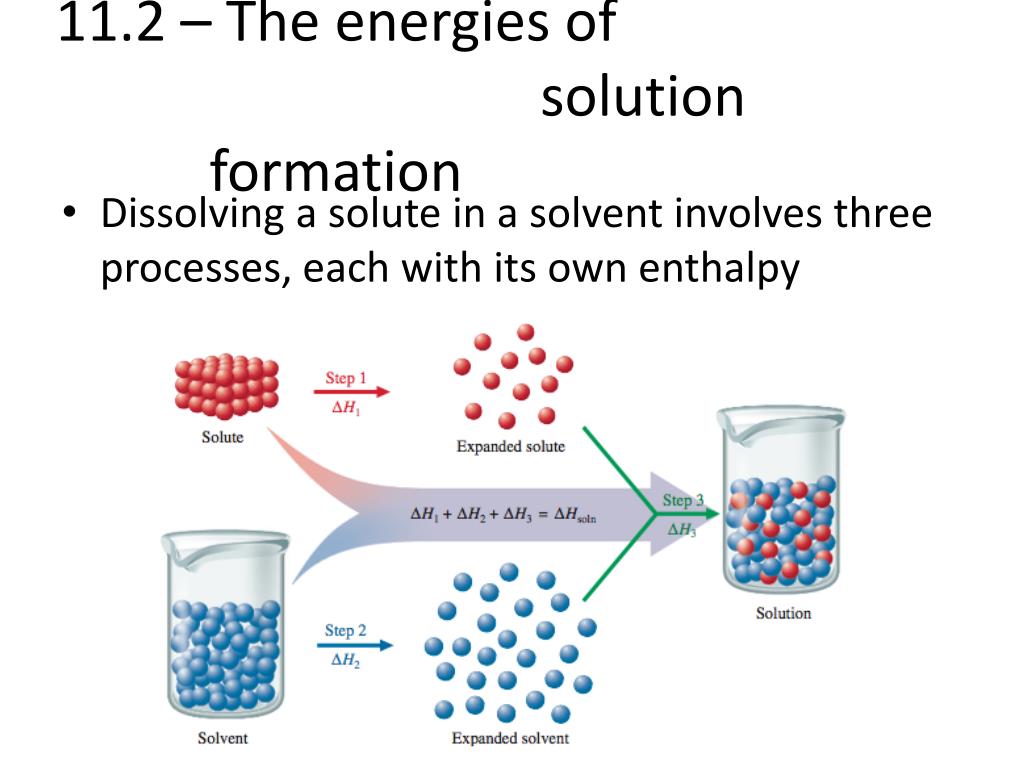
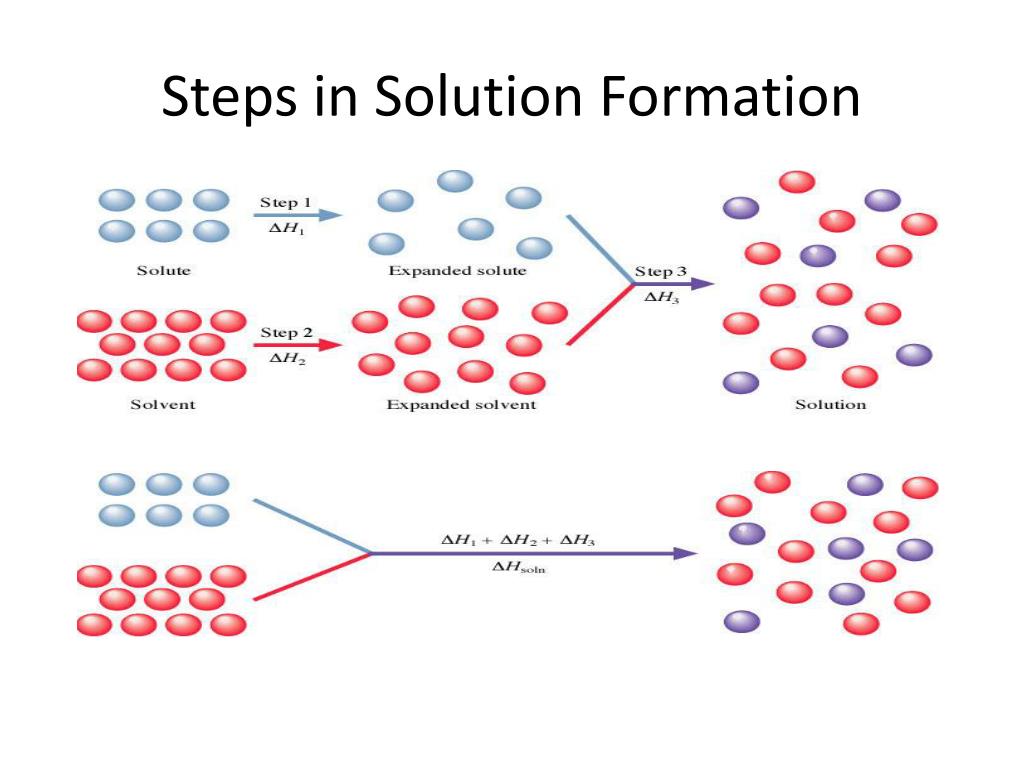
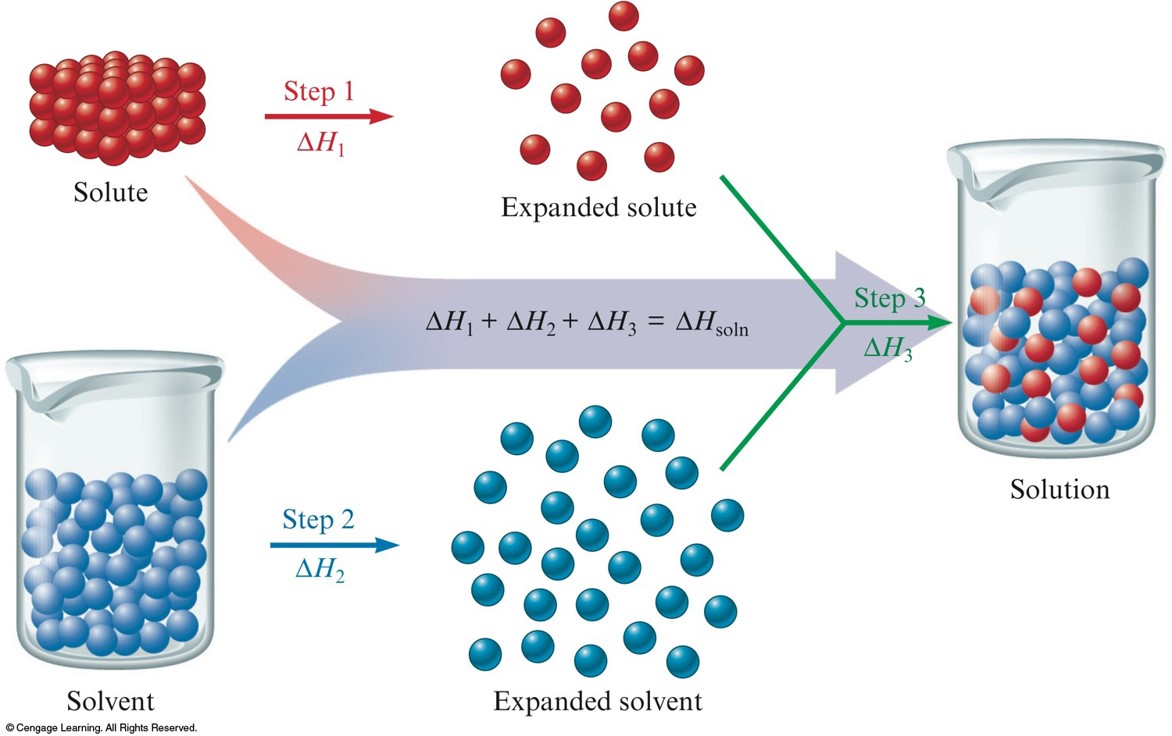
So, imagine your solid solute – let's say, salt. It's all cozy, its tiny bits, ions or molecules, holding hands super tightly. They're like a clingy group of friends at a party, all bundled up. Meanwhile, your solvent – the liquid, like water – is also doing its thing. Its little molecules are buzzing around, bumping into each other, full of energy. Think of them as the super social butterflies at the same party.
When you mix them, something epic happens. Those buzzing solvent molecules spot the tightly-held solute bits. And they're like, "Hey, you guys look a little lonely! Let us give you a whirl!" They start to nudge, prod, and pull at those solute particles.
This whole process isn't exactly a smooth, zero-effort affair. It takes energy to break those solute bonds. Think of it like trying to peel apart those clingy friends. It requires a bit of a tug. So, the solvent molecules have to use some energy to pry those solute bits away from each other.
But wait, there's more! Once those solute particles are free, they get to mingle with the solvent. And guess what? They also form new attractions. They start holding hands (or, you know, electrostatically attracting) with the solvent molecules. It's like the solute friends finally realized there are other, cooler people to hang out with at the party.
Now, here's where the energy story gets really fun. Forming these new attractions between solute and solvent releases energy. It's like a little "thank you" from the universe for making new friends. This released energy is actually pretty cool because it can help pay for the energy that was needed to break the original solute bonds.
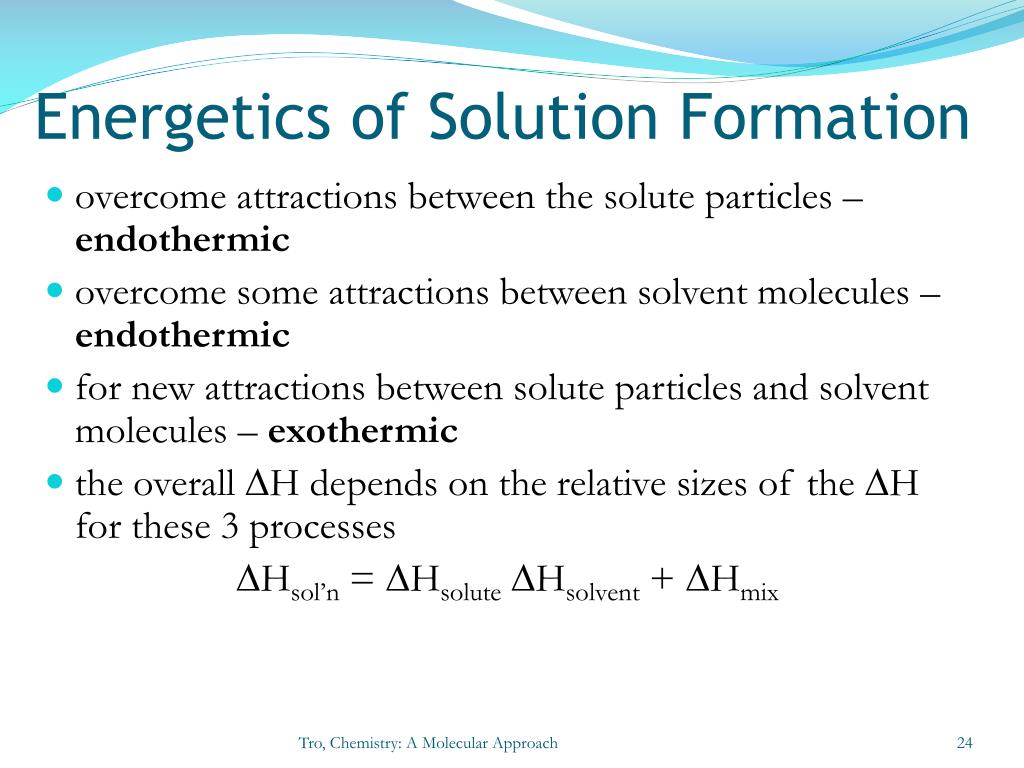
So, we've got two main energy players here: the energy needed to break solute bonds, and the energy released when solute and solvent make new pals. The overall energy change in your cup of coffee is the balance between these two. It's like a tiny energy tug-of-war.
The Three Big Possibilities
This energy tug-of-war can go in three main directions. It’s like a choose-your-own-adventure for your drink!
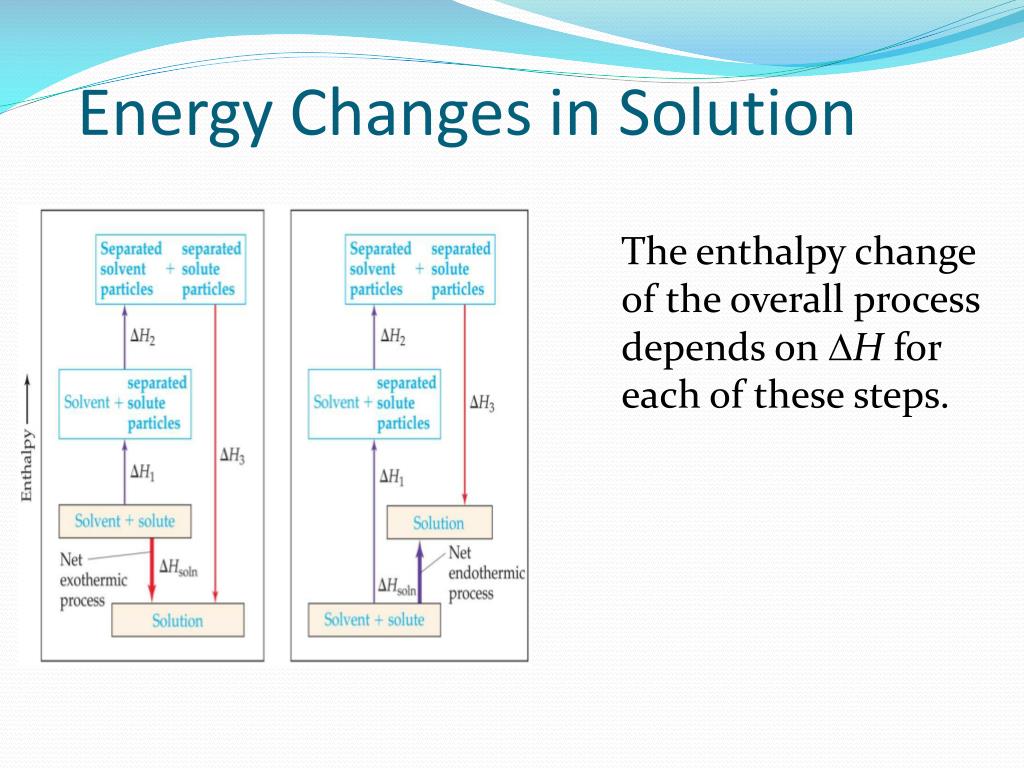
Scenario 1: The "Chill Out" Solution (Exothermic)
Sometimes, the energy released when the new solute-solvent friendships form is way more than the energy needed to break the old solute bonds. When this happens, there's extra energy left over. And what does that extra energy do? It gets released into the surroundings. This means your solution might feel warm or even hot! Think about dissolving strong acids in water – it can get seriously toasty. It's like the molecules are so excited about their new friends, they start to heat things up. Pretty neat, right?
This is called an exothermic process. "Exo" means out, and "thermic" means heat. So, heat is going out!
Scenario 2: The "Sweaty" Solution (Endothermic)
On the flip side, sometimes it takes a ton of energy to pry those solute particles apart. The energy needed to break those initial bonds is greater than the energy released when the new solute-solvent bonds are formed. So, where does that extra energy come from? You guessed it – it has to be absorbed from the surroundings. This means your solution will get colder. Ever used an instant ice pack? BAM! Endothermic reaction. The dissolving process is so busy making new friends that it's stealing heat from its environment to do it. It's like the molecules are so focused on breaking up and making new connections, they forget to share their warmth.
This is the endothermic process. "Endo" means in, and "thermic" means heat. So, heat is going in!
Scenario 3: The "Meh" Solution (Near Zero Energy Change)
And then, you have the cases where the energy needed to break the solute bonds and the energy released when new solute-solvent bonds form are pretty much equal. It’s a perfect balance. The energy change is basically zero. Your solution neither heats up nor cools down significantly. It’s like the molecules had a polite handshake and then went their separate ways. No drama, no fuss. Just a good old-fashioned dissolve.
This is the sweet spot where the energy changes cancel each other out. It’s all about that equilibrium, baby!
Why Is This So Cool? (Besides the Temperature Changes!)
Okay, so the temperature changes are fun, but there's more to this energy dance. Understanding these energy shifts helps us predict if something will even dissolve in the first place. Remember the old saying, "like dissolves like"? Well, energy plays a huge role in that. If the energy landscape is favorable – meaning the solute and solvent can form stable new bonds without using up too much energy – then dissolution happens easily.
It’s also why different substances behave so differently. Imagine trying to dissolve a rock in water. That would take a massive amount of energy to break the strong bonds holding the rock together. It's just not in the cards for water molecules to provide that much energy. But for something like sugar? Its bonds are weaker, and water is really good at forming those new attractions, making it a perfect dissolving partner.
Think about oil and water. They really don't want to mix. The energy cost of breaking water-water bonds and oil-oil bonds, and then trying to force oil and water molecules to be friends, is just too high. They’d rather stick with their own kind. It’s a molecular standoff, and energy is the ultimate deciding factor.
The formation of solutions is a beautiful illustration of the first and second laws of thermodynamics in action, all without a textbook in sight. It’s the universe doing its thing, finding the most stable, lowest-energy configuration. And sometimes, that configuration involves your salt disappearing into your soup.
So, the next time you stir something into a liquid and feel a subtle warmth or coolness, or notice nothing at all, you're witnessing a miniature energy saga. It’s the unseen forces at play, the molecular friendships being forged, and the energy being exchanged. It’s not just magic; it’s science being incredibly cool and, dare I say, a little bit playful.
And that, my friend, is what happens to energy when you make a solution. It’s a tiny, energetic party, and everyone’s invited!
