What Forces Typically Hold Nonmetal Atoms Together Within A Molecule
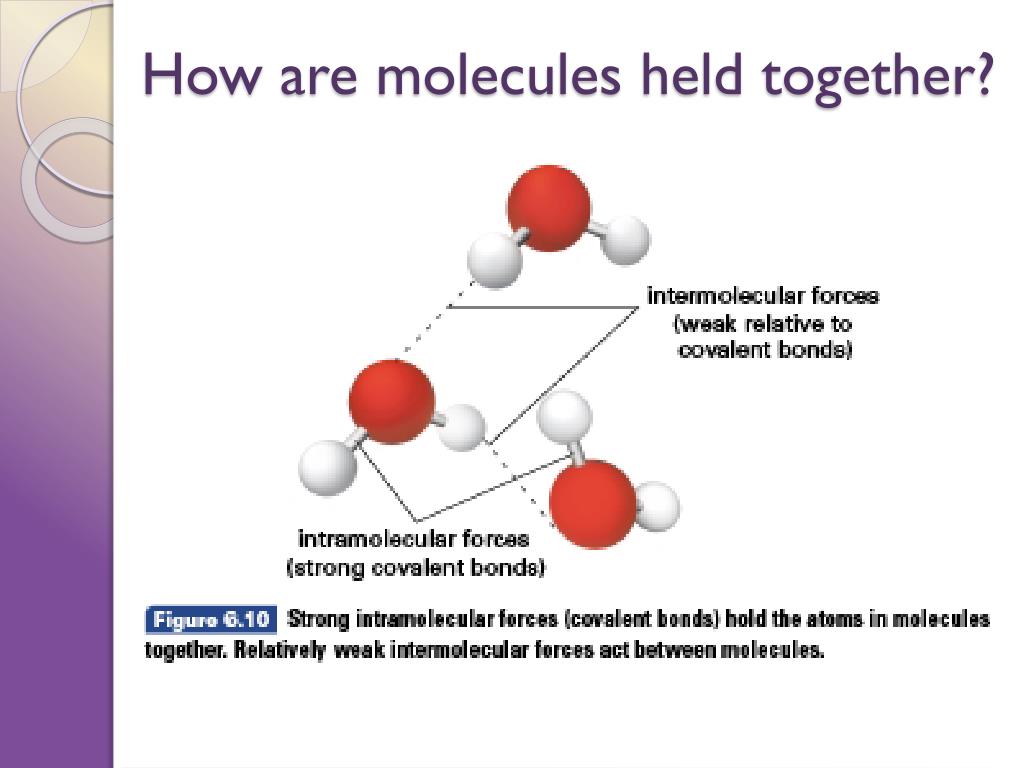
Ever wondered why water stays together as a liquid, or why the air you breathe is made of distinct molecules like nitrogen and oxygen? It's not magic, it's science! And understanding the forces that hold nonmetal atoms together within a molecule is like unlocking a secret language of the universe. It’s a fascinating peek into the tiny, invisible world that makes up everything around us, from the simplest breath to the most complex biological processes. Learning this isn't just for scientists; it’s for anyone who's ever looked at a snowflake and thought, "How does that even happen?"
The primary purpose of understanding these forces, collectively known as chemical bonds, is to explain the incredible diversity of matter. These bonds are the glue that sticks atoms together to form molecules, and it's the arrangement and type of these bonds that dictate a substance's properties. Think about it: the same atoms, carbon, can form incredibly soft graphite (think pencil lead) or incredibly hard diamonds. The difference lies entirely in how those carbon atoms are bonded! The benefits of this knowledge are vast, allowing us to predict how substances will behave, design new materials, and even understand how our own bodies function.
In education, this concept is fundamental to chemistry and physics. From high school classrooms where students first encounter Lewis structures, to university labs where researchers are synthesizing new drugs, the principles of chemical bonding are at play. In our daily lives, it's everywhere. The structure of the plastic in your water bottle, the way medicines work in your body, the flame of a candle – all are governed by these invisible forces. Even cooking involves chemical reactions driven by the breaking and forming of bonds!
So, how do these nonmetal atoms, which tend to want to grab onto electrons, decide to hold hands? For nonmetals, the most common way they bond is through covalent bonds. Imagine atoms as children wanting to share their toys. Instead of one atom completely giving an electron to another (which happens with metals), nonmetal atoms share electrons. This sharing creates a stable arrangement where both atoms feel like they have enough electrons. These shared pairs of electrons act like a strong, invisible rope, pulling the atoms together.
..jpg)
There are a few flavors of this sharing. Sometimes, atoms share electrons equally, like two equally matched tug-of-war players. This is called a nonpolar covalent bond. Other times, one atom is a bit greedier and pulls the shared electrons closer to itself. This creates a slight positive charge on one atom and a slight negative charge on the other, making it a polar covalent bond. This uneven sharing is responsible for fascinating properties, like why water molecules stick to each other, allowing insects to walk on its surface!
Curious to explore this further? You don't need a lab coat! Start by looking at the periodic table. Notice how nonmetals are grouped together. Think about everyday molecules you know – water (H₂O), carbon dioxide (CO₂), methane (CH₄). You can even find simple diagrams online showing how the electrons are shared in these molecules. Next time you see a diagram of a molecule, try to imagine those shared electrons as tiny magnets holding the atoms in place. It's a simple yet powerful way to visualize the forces that build our world.
