What Are The Customary Units Of Solubility On Solubility Curves

Ever found yourself staring at a fizzy drink, wondering why the bubbles are doing their thing? Or maybe you've experimented in the kitchen, trying to dissolve sugar in iced tea versus hot tea, and noticed a big difference? These everyday marvels are all thanks to the fascinating world of solubility, and understanding it can be surprisingly fun and useful!
Think of solubility as a substance's ability to dissolve into another substance, often a liquid. It's like a party where one ingredient is trying to mix in with another. For many of us, this concept might seem like something confined to a science lab, but the truth is, it plays a role in so many aspects of our lives, from the food we eat to the medicines we take. Understanding how things dissolve helps us optimize processes, improve product quality, and even ensure safety.
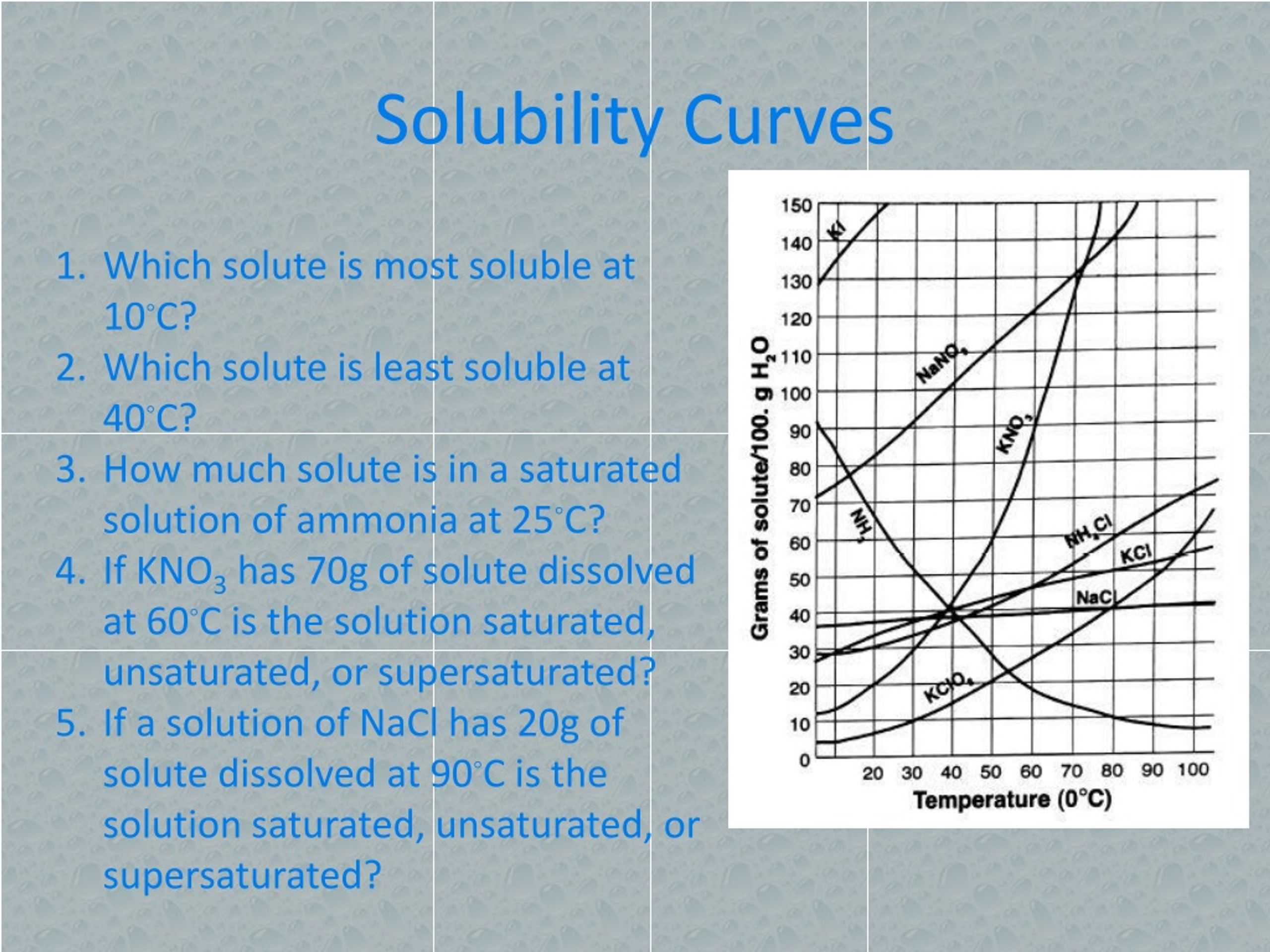
When we talk about measuring solubility, especially on those handy solubility curves you might see in textbooks or online, we're often referring to specific units. These units tell us how much of a substance can dissolve in a given amount of solvent. The most common way to express this is in grams of solute per 100 grams of solvent. So, a solubility curve might show that at a certain temperature, 30 grams of salt can dissolve in 100 grams of water. It’s a simple, yet powerful way to quantify this phenomenon.
Another frequently used unit is moles of solute per liter of solution, often represented as molarity (M). This is particularly common in chemistry where precise concentrations are crucial. You might also encounter grams of solute per liter of solution, giving you a direct measure of mass in a volume. While these might sound technical, they all serve the same purpose: to give us a clear picture of how much dissolving can happen under different conditions, especially temperature and pressure.
So, how does this apply to your daily life? Think about making a cup of coffee. Hot water can dissolve more coffee grounds than cold water, right? That's solubility in action! Or consider making rock candy. You dissolve a massive amount of sugar in hot water, and as it cools, the excess sugar comes out of solution, forming those beautiful crystals. In the pharmaceutical industry, understanding solubility is vital for ensuring medications are absorbed properly by the body. Even cleaning products rely on solubility to break down grease and grime.
To enjoy and understand solubility curves more effectively, try this: grab a couple of common substances you can easily dissolve, like sugar and salt. Heat some water to different temperatures (carefully, of course!) and see how much of each you can dissolve. You'll be creating your own miniature solubility experiments! When looking at actual solubility curves, pay attention to the shape of the line. Does it go up steeply, indicating solubility increases significantly with temperature? Or is it relatively flat? This tells you a lot about the substance. Don't be afraid to look up the solubility of everyday items; it’s a great way to connect the abstract science to the tangible world around you. It’s a journey of discovery, one dissolved particle at a time!
