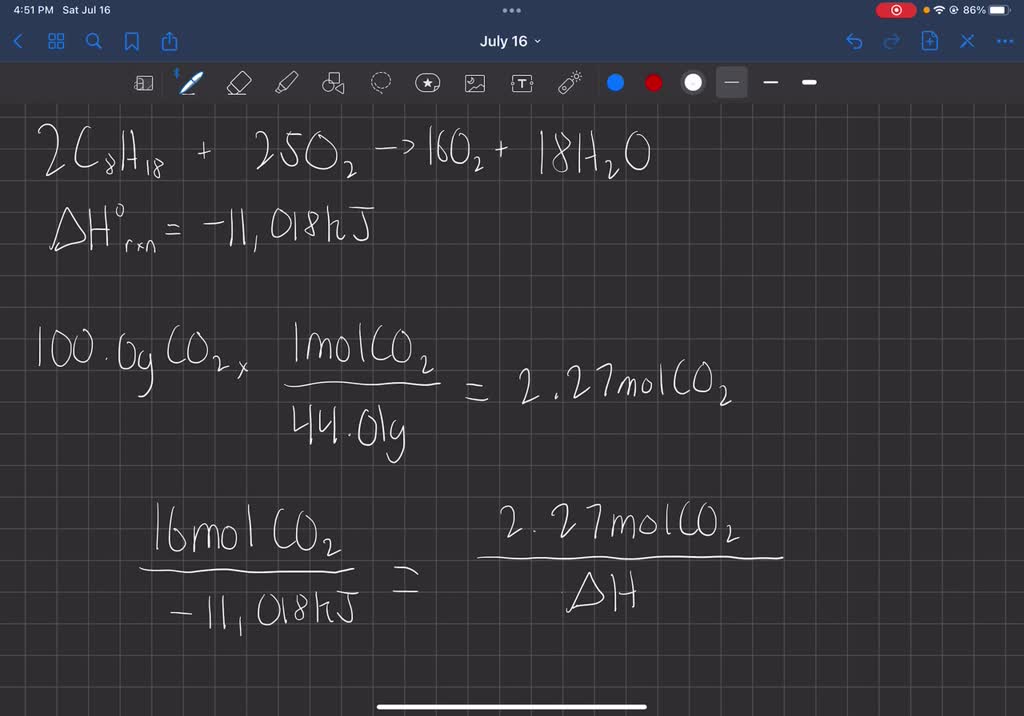
Using The Following Equation For The Combustion Of Octane

Hey there, curious cats! Ever wonder what happens when you, like, really want to get something burning? Not, like, your toast (been there, done that). I'm talking about a serious burn. The kind that powers your car. Or maybe even a rocket ship, who knows?
Today, we’re diving into the magical world of combustion. Specifically, the fiery dance of octane. You know, the stuff that makes your car go vroom.
Octane: The Star of Our Show
So, what exactly IS octane? It's a hydrocarbon. Basically, a bunch of carbon and hydrogen atoms hanging out together. Its chemical formula is C8H18. Pretty neat, right? Eight carbons, eighteen hydrogens. Like a tiny molecular party.
And this party loves to burn. Like, a lot. It’s the star ingredient in gasoline, giving it that delightful (to your engine, at least) igniting power.
The Combustion Equation: It's Not Rocket Science... Well, It Kinda Is.
Okay, so we’re gonna look at the equation for burning octane. Don't freak out! It’s actually pretty cool. Think of it as a recipe for a tiny explosion.
The equation looks like this:
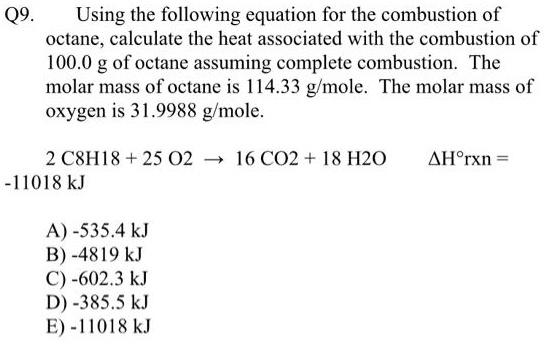
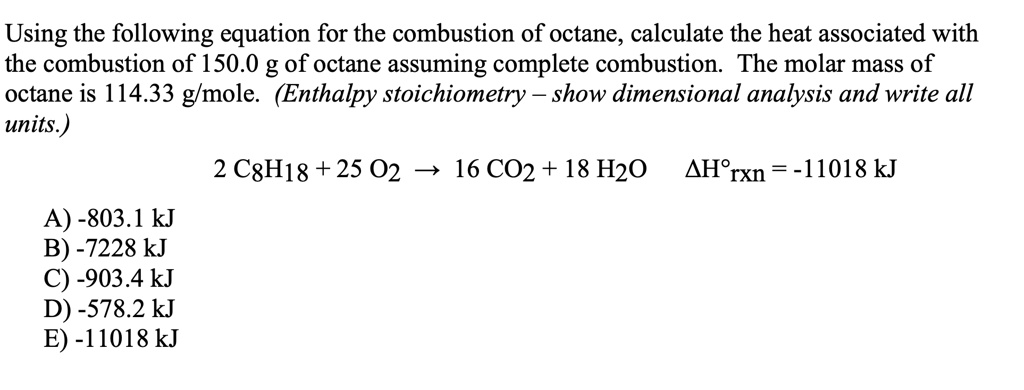
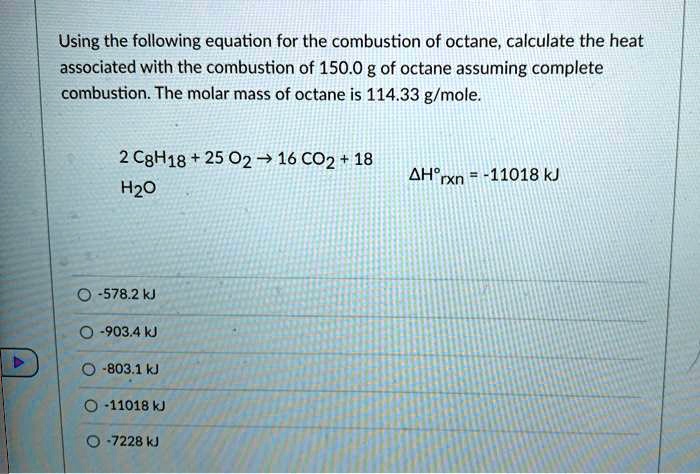
2 C8H18 + 25 O2 → 16 CO2 + 18 H2O
Whoa, sciencey! Let's break it down, buddy.
The Ingredients List
On the left side, that's our starting lineup. Our reactants. The things that are gonna get all fired up.
First up, we have 2 C8H18. That’s two molecules of octane. Imagine two little octane partygoers.
Then we have 25 O2. That’s twenty-five molecules of oxygen. Oxygen is like the super-enthusiastic friend who's always ready to jump in and get things going. Without oxygen, nothing burns. It’s the ultimate hype-person for combustion.
Think of it like this: you've got your octane fuel, and you need a good dose of air (which is full of oxygen) to make it go.
The Fiery Transformation
The arrow (→) is the "makes" part. It’s the magic wand. What happens when these two get together with a little spark?
BOOM! (Metaphorically, mostly. Unless you're talking about a car engine, then yeah, kinda literally.)
The Delicious Products
Now for the fun stuff. What do we get after the octane and oxygen have their epic showdown?
We get 16 CO2. That's sixteen molecules of carbon dioxide. Yep, the same stuff you exhale! And the stuff that makes fizzy drinks bubbly. It's also a greenhouse gas, so, you know, use responsibly.
And then we have 18 H2O. That's eighteen molecules of water. Pretty cool, huh? You take something flammable and you get water out of it. It's like alchemy, but with less wizards and more explosions.
So, octane and oxygen combine to create carbon dioxide and water. That's the simplified, ideal scenario, anyway. In the real world, there can be other byproducts, but this is the classic recipe.
Why Is This So Freakin' Cool?
Okay, besides the fact that it makes your car run, why should you care about this equation? Because it's the fundamental process behind so much of our modern world!
Think about it. Transportation relies on this. Planes, trains (well, some), and definitely automobiles. It’s the invisible force that moves us around.
And it’s not just cars. Many industrial processes use combustion. Power plants, for example, might burn fossil fuels (which contain hydrocarbons like octane) to generate electricity. So, the light bulb above your head? Might have a little octane combustion to thank.
Quirky Facts and Fun Details
Did you know that the "octane rating" in gasoline actually relates to how well the fuel resists knocking in an engine? Higher octane means better resistance. So, when you see "93 octane" at the pump, it means it's a higher-quality fuel for engines that need it.
It’s kind of like a superhero ranking for your fuel. Some engines are superheroes that can handle anything, and others are a bit more delicate and need that high-octane juice.
Also, the amount of energy released during this combustion is massive. That's why we use it! It's a very efficient way to store and release energy. It's like a tiny, controlled explosion that keeps on giving.
Imagine all those little octane molecules, just waiting for their chance to party with oxygen. It’s a beautiful, energetic chaos.
The "Why" Behind the Numbers
Let's talk about those weird numbers in front of everything. Those are called coefficients. They're crucial for something called balancing the equation.
Basically, you can’t just make stuff out of thin air. Atoms are conserved. So, the number of carbon atoms going in must equal the number of carbon atoms coming out. Same for hydrogen and oxygen.
Let’s check: * Carbon (C): We start with 2 molecules of C8H18, so that’s 2 * 8 = 16 carbons. On the product side, we have 16 CO2, which is 16 * 1 = 16 carbons. Nailed it! * Hydrogen (H): We start with 2 molecules of C8H18, so that’s 2 * 18 = 36 hydrogens. On the product side, we have 18 H2O, which is 18 * 2 = 36 hydrogens. Perfect! * Oxygen (O): We start with 25 molecules of O2, so that’s 25 * 2 = 50 oxygens. On the product side, we have 16 CO2 (16 * 2 = 32 oxygens) PLUS 18 H2O (18 * 1 = 18 oxygens). So, 32 + 18 = 50 oxygens. We're basically chemists now!
It's like a cosmic accounting system. Everything has to balance out. It's the universe's way of saying, "No free lunches, folks!"
More Than Just Fuel
While octane is most famous for powering our cars, the principles of combustion apply to so many things. Burning wood in a fireplace? Similar concept. Fireworks? Definitely combustion, and way more colorful!
It's a fundamental chemical reaction that's both powerful and, in its own way, elegant. It transforms simple substances into new ones, releasing energy in the process.
The Takeaway
So, next time you hear the roar of an engine, or even just light a candle, remember the tiny, energetic party happening at the molecular level. Remember octane, the hydrocarbon superstar, and its fiery dance with oxygen.
It's a little bit of science, a lot of energy, and a whole lot of what makes our world go 'round. Pretty cool for a bunch of atoms, right?
