Understanding Conceptual Components Of The Enthalpy Of Solution

Ever found yourself staring into a fizzy drink, wondering what's really happening when that sugar cube or salt crystal disappears? Or maybe you've been making some homemade ice cream, and noticed how things get surprisingly chilly? Well, my friends, you're about to dive into a secret superpower of the universe: enthalpy of solution! Don't let the fancy name scare you; it's actually a super cool concept that explains why things dissolve the way they do, and it can totally make your everyday life a little more scientific and a lot more fun.
So, what is this "enthalpy of solution" thingy? Think of it as the energy story that unfolds when you toss something solid, like salt or sugar, into a liquid, usually water. It’s all about the energy changes that go on behind the scenes. When we talk about enthalpy, we're essentially talking about heat – specifically, how much heat is either released or absorbed during a process.
Now, dissolving isn't just a simple act of things vanishing, is it? There are actually a few key players involved in this grand dissolution drama. Let's break them down into bite-sized, easy-to-digest pieces. Prepare to be amazed!
The Breaking Up is Hard to Do (and Costs Energy!)
First up, we have the lattice energy. Imagine your salt crystal as a tiny, perfectly organized city of ions (charged atoms), all held together by strong electrostatic forces. It's like a super-tight hug! To get those ions to break free and go their separate ways, you need to put in some serious energy. This is like un-hugging everyone in the city. It takes effort, right? So, the lattice energy is the energy required to break apart the crystal lattice of the solute (the stuff you're dissolving).
Think of it as the energy cost of getting the party started. This part is always, always endothermic, meaning it requires energy to happen. The stronger the forces holding the lattice together, the higher the lattice energy will be. So, some things are harder to pull apart than others, just like some friendships are tougher to dissolve!
Water, Water Everywhere, and It's Ready to Mingle!
Next, we need to consider what the solvent (usually water) is up to. Water molecules are pretty neat, aren't they? They're like tiny magnets, with a positive end and a negative end. When you add your solute ions, water molecules get super excited to interact with them. This is called hydration energy.
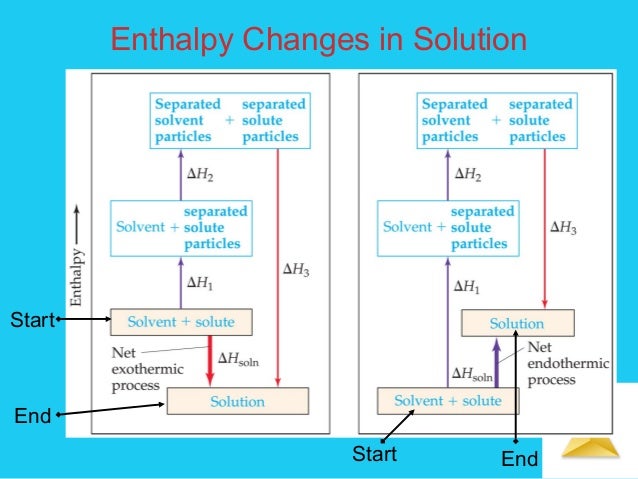
The positive ends of water molecules will surround the negative ions, and the negative ends will surround the positive ions. It's like water molecules forming little cozy blankets around each ion, preventing them from getting back together. This process, where the solvent molecules surround the solute particles, releases energy. It’s a happy reunion!
This hydration energy is always exothermic, meaning it gives off energy. The more attracted the ions are to water, the more energy is released. So, some ions are absolute social butterflies and get a huge energy hug from water!
The Big Finale: The Net Energy Change!
So, we've got the energy needed to break apart the solute (lattice energy, endothermic) and the energy released when water hugs those broken-apart bits (hydration energy, exothermic). The enthalpy of solution is simply the sum of these two energies!
It's like adding up your expenses and your income for the day to see if you're in the red or in the green. If the energy released by hydration is greater than the energy needed to break the lattice, the overall process is exothermic. This means the solution gets warmer!

Think about dissolving sodium hydroxide (lye) – it gets super hot! That's because the hydration energy is much larger than the lattice energy. It’s like the water giving a super-duper energetic hug.
On the other hand, if the energy needed to break the lattice is greater than the energy released by hydration, the overall process is endothermic. This means the solution gets colder! Remember that cool pack you might use for an injury? Those often work by dissolving a salt like ammonium nitrate in water, which makes the pack feel refreshingly cold.
It’s like the water’s hug isn’t quite enough to compensate for the effort of breaking up the crystal. The system actually draws heat from its surroundings to make the process happen, hence the cooling effect. How neat is that?
Why Should You Care About This Scientific Shenanigan?
Okay, okay, you might be thinking, "That’s all well and good, but how does this make my life fun?" Oh, my friends, the fun is in the understanding!
Imagine you’re baking and a recipe calls for dissolving something. Knowing about enthalpy of solution can explain why your batter might be slightly warm or cool. It's a little peek into the molecular dance happening in your kitchen!
Or consider a hot summer day. You’re making lemonade, and you want the sugar to dissolve quickly. Understanding that some substances dissolve endothermically (making things colder) and some exothermically (making things warmer) can be your secret weapon. Perhaps you want a drink that’s instantly cooler? (Though most common solutes will primarily cause a slight temperature change, the principle is there!).
It’s about seeing the world around you with a little more wonder. It’s about appreciating the invisible forces at play. Every time you dissolve something, you’re witnessing a tiny scientific experiment. You’re participating in the fascinating world of chemistry!

This concept also helps us understand why certain substances dissolve well in water and others don't. It's all about the balance between breaking those solute bonds and forming new solvent-solute interactions. It’s a constant negotiation of energy!
The Joy of Discovery Awaits!
So, the next time you add sugar to your tea or salt to your pasta water, take a moment. Think about the energy being exchanged. Think about those tiny ions breaking free and the water molecules rushing in to embrace them. It’s a tiny ballet of attraction and repulsion, a constant give-and-take of energy.
Understanding the conceptual components of enthalpy of solution isn't just about memorizing formulas; it's about unlocking a deeper appreciation for the world. It’s about turning everyday occurrences into moments of scientific curiosity and delight.
This is just the tip of the iceberg, of course! There’s a whole universe of chemistry waiting to be explored, and it’s all around you. So, go forth, be curious, and let the adventure of understanding the world, one dissolving particle at a time, inspire your journey!
