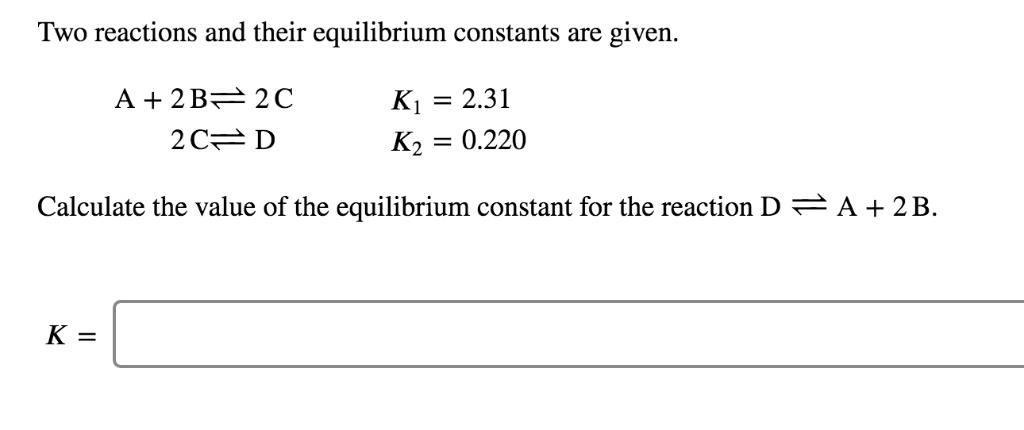
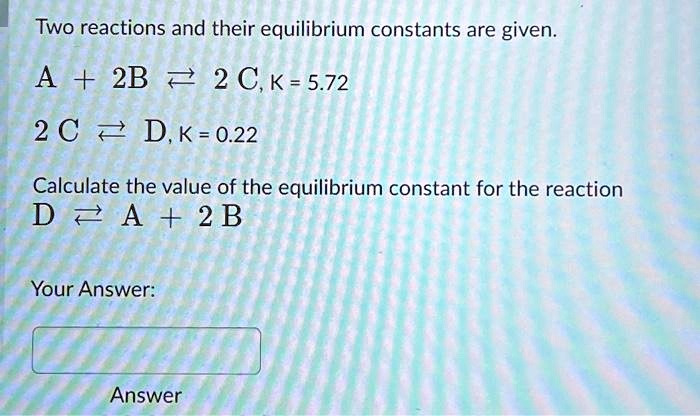
Two Reactions And Their Equilibrium Constants Are Given.

Imagine you're at a bustling party, and two amazing things are happening simultaneously. In one corner, everyone's doing the Cha-Cha Slide with infectious energy, while in another, a quiet, but equally captivating, group is engaged in a sophisticated game of chess. Both are popular, both are enjoyable, but they're clearly very different vibes, right?
Well, in the quirky world of chemistry, reactions can be a lot like these party scenarios. Sometimes, they're wild and energetic, like our Cha-Cha Slide dancers, while other times, they're more measured and strategic, like the chess players. And just like you might want to know which party activity is drawing more people, scientists like to understand how much of each reaction is "happening" at any given moment. This is where our star players, equilibrium constants, come in.
Let's dive into a little story about two imaginary reactions that are just trying to find their balance, like everyone at our party. We'll call them Reaction A and Reaction B. Now, these aren't just any reactions; they're the stars of our show, and we've been given a secret code for each of them: their equilibrium constant.
Think of the equilibrium constant as a scorekeeper. It tells us, at a certain temperature (let's pretend it's a perfect 25 degrees Celsius, nice and comfy!), how much of the "done" stuff (the products) there is compared to the "starting" stuff (the reactants) when the reaction has settled down and isn't visibly changing anymore. It's like the party has reached a point where the Cha-Cha dancers are still dancing, and the chess players are still playing, and the number of people doing each isn't really changing. Everything's in its own kind of balance.
Now, for Reaction A, its equilibrium constant is a rather modest 10. What does this mean? Well, it's like our Cha-Cha Slide corner. When the music finally settles down and the reaction reaches its "equilibrium," there's a good amount of the "done" stuff, but there's also still a decent chunk of the "starting" stuff hanging around. It's a lively scene, with both sides contributing to the party. The dance floor isn't completely empty of original dancers, but a good number have definitely joined in the Cha-Cha fun.

On the other hand, we have Reaction B, and its equilibrium constant is a whopping 1,000,000! Whoa! That's a big number. This is like the chess corner, but with a twist. When Reaction B settles down, it's almost entirely "done." The chess players have mostly finished their games, and the "done" pieces (the products) are everywhere. It’s like the chess players got so engrossed that they’ve basically completed every game imaginable, leaving very few starting pieces left untouched. They've embraced the chess spirit so wholeheartedly that the game is virtually "finished" in its most complete form.
So, we have two very different equilibrium constants, telling us two very different stories about our reactions. Reaction A is like a popular dance floor where people are still mingling and doing their thing, with a healthy mix of participants. It's got enthusiasm, but it's not an overwhelming tide of one thing.
And Reaction B? That’s like a highly focused, almost single-minded pursuit of perfection. When it reaches its equilibrium, it’s almost entirely dedicated to the "done" state. It’s so good at becoming its "done" version that it leaves very little of its "starting" self behind. It's a testament to its incredible efficiency, like a masterful artist who doesn't leave a single brushstroke undone.
Isn't it fascinating? These simple numbers, the equilibrium constants, give us a peek into the heart of these chemical reactions. They tell us whether a reaction is a bit of a social butterfly, happy to have a mix of reactants and products, or a dedicated master of its craft, leaning heavily towards becoming something new. They're not just abstract numbers; they're like little stories of how things balance out in the microscopic world, a world that, when you think about it, is constantly playing out its own amazing party.
So, next time you hear about an equilibrium constant, don't just think of a boring number. Think of a bustling party, a dedicated game, or a passionate artist. Think about how different things can reach their own unique state of balance, each with its own special kind of success. It’s all about finding that sweet spot, whether it’s a lively dance or a silent, triumphant game.
