Two Organic Functional Groups In A Phospholipid Molecule

Hey there, science explorer! Ever wonder what makes up those tiny, amazing building blocks of life, like cell membranes? Well, today we're diving into the world of phospholipids. Think of them as the VIP guests at the cell party, and we're going to zoom in on two of their super-cool organic functional groups. No need to grab a textbook; we're doing this the fun way!
So, what exactly are these phospholipids? Imagine a tiny tadpole. That's kind of what a phospholipid looks like! It has a head and two tails. The head is all about being friendly with water – we call that hydrophilic. The tails, on the other hand, are a bit shy around water and prefer to hang out with other fatty molecules – they're hydrophobic. This dual personality is what makes them perfect for forming cell membranes. They're like the ultimate social butterflies, but with very specific social rules!
Now, let's talk about those functional groups. These are like the special features or attachment points on our tadpole molecule. They're what give the phospholipid its unique properties and allow it to do its job so brilliantly. And today, our stars of the show are:
The "Oh So Phosphorylated" Phosphate Group!
First up, we have the phosphate group. This guy is a real powerhouse. Chemically, it's a phosphorus atom bonded to four oxygen atoms. Sounds a bit like a fancy science recipe, right? But what makes it special is its negative charge. Yep, this little group is rocking a bit of an electrical vibe!
Think of it like this: if the phospholipid were a tiny character, the phosphate group would be its incredibly friendly, outgoing personality. It’s always ready to interact with its watery surroundings. It’s like the part of the molecule that loves a good splash!
This negative charge is a big deal. It's what makes the "head" of our phospholipid tadpole so attracted to water. Water molecules themselves have a bit of a positive and negative side, and opposites attract, as they say! So, the phosphate group is like a tiny magnet, pulling water molecules in. It’s the reason why phospholipids can form structures in water, like the lipid bilayer that makes up our cell membranes.
It’s not just about being a friend to water, though. The phosphate group is also the connector. It’s the crucial link between the fatty acid tails and the rest of the phospholipid head. Imagine it as the sturdy bridge holding everything together, ensuring our tadpole stays in one piece while it’s busy doing its membrane-forming duties.
And here’s a fun little tidbit: the "phospho" in phospholipid actually comes from this very group! It's like the group is so important, it got to name a whole class of molecules. Talk about having a big ego… or just being really, really essential!
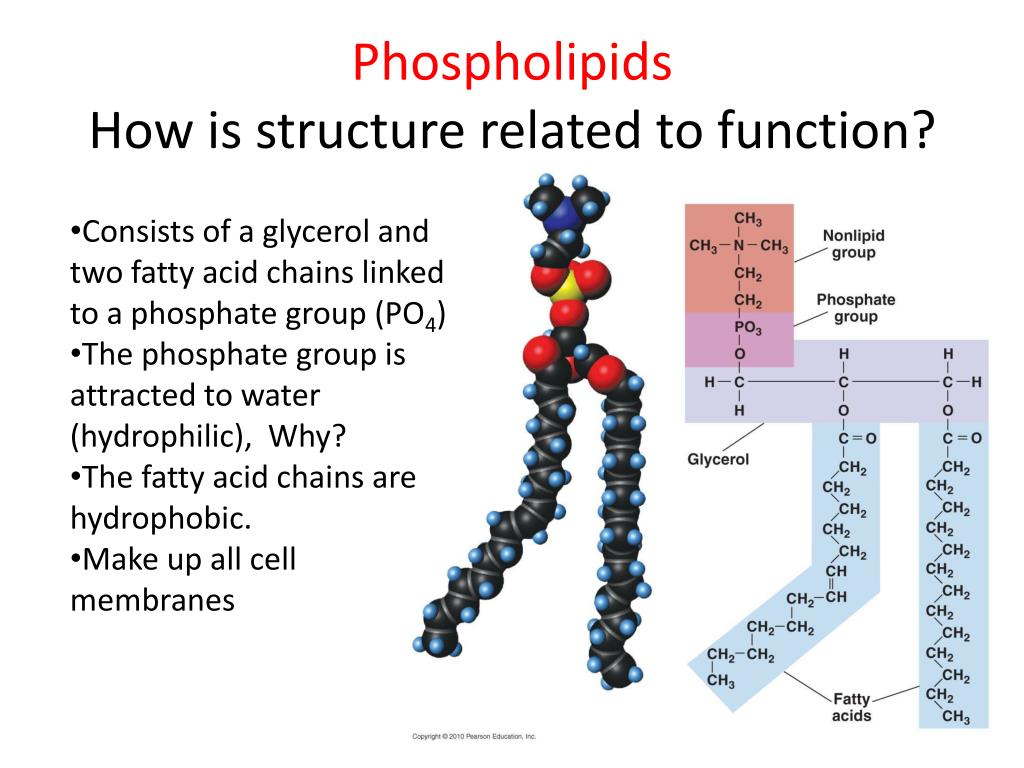
The structure of the phosphate group is also quite interesting. It has this tetrahedral arrangement, meaning the oxygen atoms are pointing towards the corners of a tetrahedron shape around the central phosphorus atom. It’s like a little 3D starburst of negativity, always ready to party with polar molecules.
But wait, there's more! The phosphate group itself can often have other small molecules attached to it. These are called "head groups," and they can vary quite a bit. Think of them as different hats the phosphate group can wear, further customizing the phospholipid. Some of these head groups are also charged, adding even more to the water-loving nature of the head. Others are a bit more neutral but still contribute to the overall polarity. It's like having a whole collection of accessories for our phosphate friend!
This variability in head groups is what leads to different types of phospholipids, each with its own specific role. Some might be better at stabilizing membranes, others might be involved in signaling. It’s a whole diverse family, all stemming from that foundational phosphate group.
So, to sum up our phosphate friend: it’s negatively charged, loves water, acts as a vital connector, and even gives phospholipids their name. It’s a small group, but it punches way above its weight in terms of importance! Give a little cheer for the phosphate group – it’s a true unsung hero of the cellular world!
The "Glycerol: The Backbone's Got Your Back!" Group
Next on our tour of phospholipid wonders is the glycerol group. Now, glycerol might sound a bit like a fancy dessert topping, and in a way, it kind of is – a sweet, sweet backbone for our phospholipid tadpole! Chemically, it’s a simple sugar alcohol. Think of it as a three-carbon chain, with each carbon atom happily holding onto a hydroxyl group (-OH).
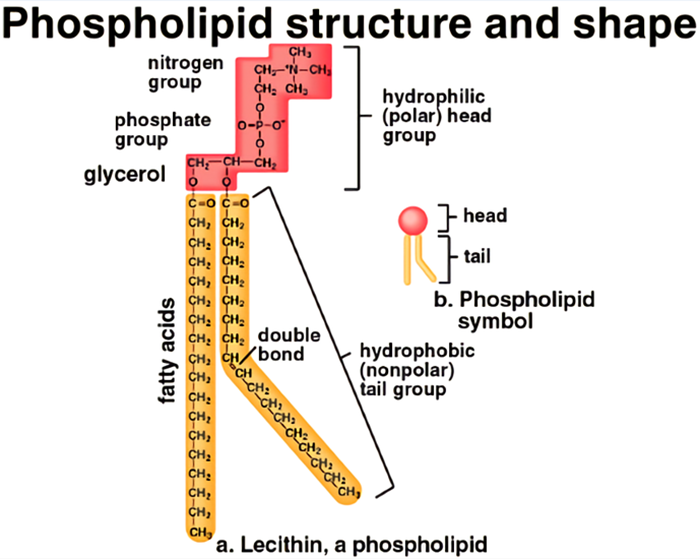
This glycerol molecule is the central hub, the main stage where all the action happens. It's where the fatty acid tails attach, and it's also where our friend the phosphate group gets to anchor itself. It’s the literal foundation of our phospholipid structure.
Remember our tadpole analogy? The glycerol is like the body of the tadpole, the part that connects the head (phosphate group) to the tail (fatty acid chains). It's stable, it's reliable, and it’s crucial for keeping the whole molecule together.
The hydroxyl groups on glycerol are important because they provide the attachment points. They're like little hands ready to grab onto other molecules. Two of these hydroxyl groups will be busy forming ester bonds with the fatty acid tails. And the third hydroxyl group? That's the one that gets to link up with our phosphate group, creating the phospholipid head.
Glycerol itself is a fairly polar molecule due to those hydroxyl groups. This means it has a bit of a positive and negative charge distribution, making it comfortable in watery environments. So, while the fatty acid tails are doing their best to hide from water, the glycerol part of the molecule is quite happy to be near it, bridging the gap between the hydrophilic and hydrophobic regions.
It’s a humble molecule, glycerol. It doesn't have a dramatic charge like the phosphate group, but its structural role is absolutely indispensable. Without glycerol, phospholipids wouldn’t have that organized structure that allows them to form membranes. Imagine trying to build a house without a foundation – chaos! Glycerol is that strong, reliable foundation.
And here’s a cool fact: glycerol is actually used in lots of other things too! It’s found in foods for sweetness and moisture, in cosmetics for moisturizing, and even in some pharmaceuticals. So, this little backbone molecule is a bit of a celebrity in its own right!

The way glycerol connects the fatty acids is also pretty neat. It forms what are called ester linkages. These are strong chemical bonds that hold the long fatty acid chains securely to the glycerol backbone. It’s like the fatty acids are zipped up tight onto their central support structure.
This stability provided by the glycerol backbone is essential for the integrity of cell membranes. It ensures that the membrane doesn’t just fall apart at the slightest disturbance. It’s a testament to the elegance of biological design that such simple molecules can create such robust and functional structures.
So, while the phosphate group gets all the glory for its water-loving ways and its negative charge, let's not forget the unsung hero, the steady backbone: glycerol! It’s the quiet achiever, the reliable support system that makes it all possible. Cheers to glycerol!
Putting It All Together: The Phospholipid Party!
Now that we’ve met our two star functional groups, let’s see how they team up. The glycerol acts as the central connector. On two of its arms, it holds onto those hydrophobic fatty acid tails – the part of the phospholipid that’s like, “Nope, not talking to water, thanks!”
On its third arm, the glycerol is linked to the phosphate group. And this phosphate group, with its awesome negative charge, is the reason the "head" of the phospholipid is so wonderfully hydrophilic. It's like the phosphate group is saying, "Water! My favorite! Come over here, everyone!"

So, you have this molecule that’s literally designed to be half water-lover and half water-avoider. When these phospholipids get together in a watery environment (like inside and outside our cells), they naturally arrange themselves into a bilayer. The hydrophilic heads all face outwards, towards the water, and the hydrophobic tails huddle together in the middle, away from the water.
It’s like a perfectly orchestrated dance party! The heads are mingling with the water, and the tails are having their own little hydrophobic rave in the center. This lipid bilayer is the fundamental structure of every cell membrane, acting as a barrier that controls what enters and leaves the cell. It’s a pretty incredible feat for a tiny molecule!
The specific arrangement and the nature of the functional groups are what give cell membranes their unique properties – their fluidity, their ability to repair themselves, and their selective permeability. It’s a testament to how even the smallest organic molecules can have profound impacts on the larger picture of life.
So, the next time you think about cells or membranes, give a little nod to the phosphate group and the glycerol. They’re the dynamic duo, the foundation and the flair, that make it all happen. They are the unsung heroes, working tirelessly to keep our cells in tip-top shape.
Isn’t it amazing how these simple organic groups come together to create something so vital for life? It’s like the universe’s way of showing us that even the smallest components can have the biggest roles. So, keep exploring, keep questioning, and remember that there’s a whole world of wonder in every tiny molecule!
And with that, I hope you’re feeling a little more enlightened and a lot more cheerful about the building blocks of life. Go forth and share your newfound phospholipid wisdom! You’ve got this!
