To Which Third Period Element Do These Ionization Values Belong
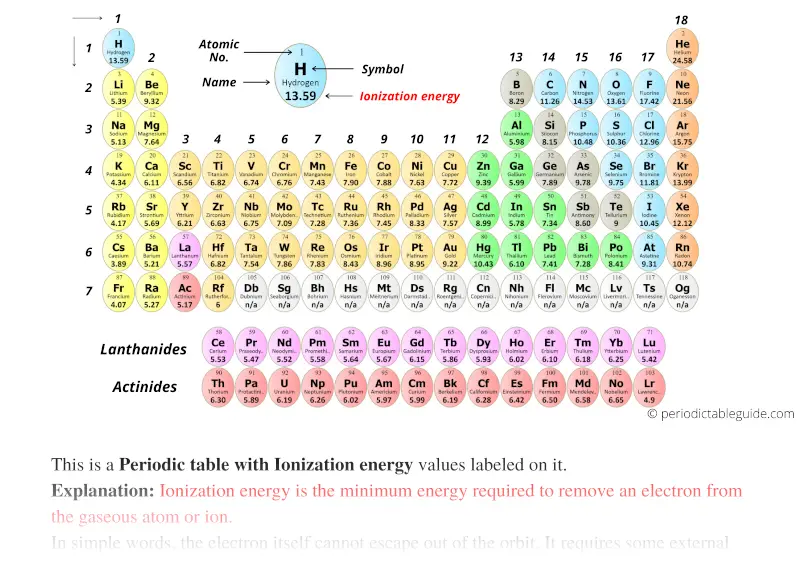
Hey everyone! Ever looked at a bunch of numbers and felt a little … mystified? Like, what do they even mean? Today, we're diving into a cool little puzzle from the world of chemistry, and trust me, it's way less intimidating than it sounds. We're going to play detective and figure out which element, from the third period of the periodic table, is hiding behind some interesting ionization energy values.
So, what's a "third period element" anyway? Think of the periodic table like a big, organized apartment building for all the atoms out there. Each floor is a "period," and the third floor is where we're going to be snooping around. These are elements that have their electrons arranged in three main energy levels, kind of like how your phone has apps on three different screens.
And "ionization energy"? That's just a fancy term for how much oomph you need to give an electron to yank it right out of an atom. Imagine the electron is clinging to the atom like a kid to their favorite toy. Ionization energy is the amount of persuasive power (or energy) you need to get that kid to let go.
We've got a set of numbers here that represent the energy needed to remove successive electrons. This means we're looking at how easy it is to take away the first electron, then the second, then the third, and so on. It's like seeing how many times you can convince that kid to share their toy before they really put their foot down!
The Mystery Numbers
Here are the ionization energy values (in kilojoules per mole, kJ/mol) we're working with. Don't let the numbers themselves scare you; we're going to focus on the trends they show. It's all about the story these numbers tell!
- 1st Ionization Energy: 496 kJ/mol
- 2nd Ionization Energy: 4562 kJ/mol
- 3rd Ionization Energy: 6910 kJ/mol
- 4th Ionization Energy: 9543 kJ/mol
- 5th Ionization Energy: 13351 kJ/mol
- 6th Ionization Energy: 16613 kJ/mol
- 7th Ionization Energy: 20117 kJ/mol
Now, let's take a gander at these values. What jumps out at you? We've got this first number, 496, which seems pretty reasonable, right? Not too high, not too low. It's like getting a casual "okay, I'll share" from our imaginary kid.
But then… BAM! The second ionization energy shoots up to 4562 kJ/mol. That's a huge jump! It's like our kid went from a gentle nudge to a full-blown, "NO WAY, THIS IS MINE FOREVER!" kind of defiance. This massive leap is our biggest clue. It tells us something really important happened after we removed that first electron.
And it doesn't stop there. The subsequent ionization energies also keep climbing, and they keep climbing by pretty significant amounts. It’s like each time we try to take another electron, the atom is saying, "Oh, you think that was hard? Try this!"
Why Such a Big Jump?
So, why the dramatic increase in energy needed to remove that second electron? This is where the magic of atomic structure comes into play. Atoms are organized with electrons in different "shells" or "energy levels." Think of it like layers of an onion. The electrons in the outermost shell are the easiest to grab, the ones we call "valence electrons." They're like the fruit on the very edge of the tree – easier to reach.
When we remove the first electron, we're taking away one of these relatively easy-to-reach valence electrons. Our first ionization energy reflects this. It's a good measure of how strongly that outermost electron is held.
But once we've taken that first one, we've exposed the next layer of electrons. These electrons are in a shell that's closer to the nucleus, the positively charged heart of the atom. Because they're closer to the positive charge, they're held much, much more tightly. It's like trying to pull a toy away from a kid who's now hugging it with both arms and standing right next to their parent.

That humongous jump between the 1st and 2nd ionization energies tells us that we removed a valence electron, and the next electron we tried to remove was from a much more stable, inner shell. This means the atom we're looking at has a significantly different number of electrons in its outermost shell compared to the shell just inside it.
Let's Talk About Third Period Elements
We're focusing on the third period, remember? These elements have their electrons filling up to the third energy level. Let's list them out and see what's going on with their outer electrons:
- Sodium (Na): 1 valence electron (in the 3rd shell)
- Magnesium (Mg): 2 valence electrons (in the 3rd shell)
- Aluminum (Al): 3 valence electrons (in the 3rd shell)
- Silicon (Si): 4 valence electrons (in the 3rd shell)
- Phosphorus (P): 5 valence electrons (in the 3rd shell)
- Sulfur (S): 6 valence electrons (in the 3rd shell)
- Chlorine (Cl): 7 valence electrons (in the 3rd shell)
- Argon (Ar): 8 valence electrons (in the 3rd shell)
Now, let's consider what happens when we start removing electrons from these. If an element has only one valence electron, like Sodium, removing that first electron is relatively easy. But the second electron we try to remove will be from the second shell, which is much, much more tightly held. This would cause a huge jump between the 1st and 2nd ionization energies.
What about Magnesium? It has two valence electrons. So, the first two ionization energies would be relatively low. But the third electron we try to remove would be from that inner, second shell, and that's when we'd see a massive jump.

And Aluminum? It has three valence electrons. The first three ionization energies would be lower, and then the fourth would show that big jump.
The Smoking Gun!
Looking back at our mystery numbers:
- 1st Ionization Energy: 496 kJ/mol (relatively low)
- 2nd Ionization Energy: 4562 kJ/mol (HUGE jump!)
This pattern – a low first ionization energy followed by an enormous leap for the second – is a dead giveaway. It means the element has only one valence electron. Once that single, easy-to-remove electron is gone, we're trying to pull an electron from a much more stable inner shell, requiring a lot more energy.
Which of our third-period elements has just one valence electron in its outermost (third) shell? It's Sodium (Na)!
Sodium is an alkali metal, and like all alkali metals, it's very reactive because it's eager to get rid of that single valence electron to achieve a stable electron configuration. It's like it's saying, "Here, take this one! I'm much happier without it!"
The subsequent ionization energies (3rd, 4th, 5th, etc.) for Sodium continue to rise because we're progressively removing electrons from deeper and deeper shells, which are all more tightly bound to the nucleus. But that initial, dramatic increase between the first and second ionization energies is what really points us to Sodium.
Why is this Cool?
It's pretty neat, right? These seemingly random numbers are actually a fingerprint of an element's atomic structure. They tell us a story about how its electrons are arranged and how strongly they're held. It’s like deciphering a secret code that reveals the element's personality!
Understanding ionization energies helps chemists predict how elements will react. Elements with low ionization energies, like Sodium, tend to lose electrons easily and form positive ions. This is why Sodium is so common in compounds like table salt (Sodium Chloride)! It readily gives up its electron to Chlorine.
So, next time you see a series of ionization energy values, don't just see numbers. See a story, a puzzle, and a peek into the fascinating, hidden world of atoms. We cracked the case, and our mystery element is the one and only Sodium!
