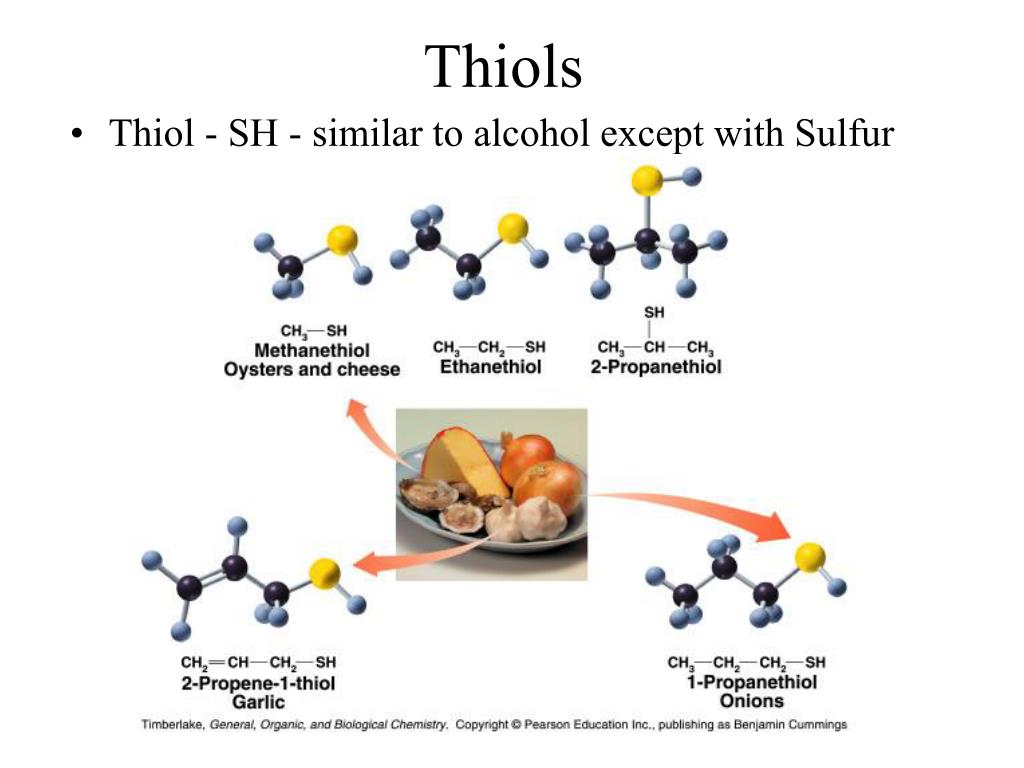
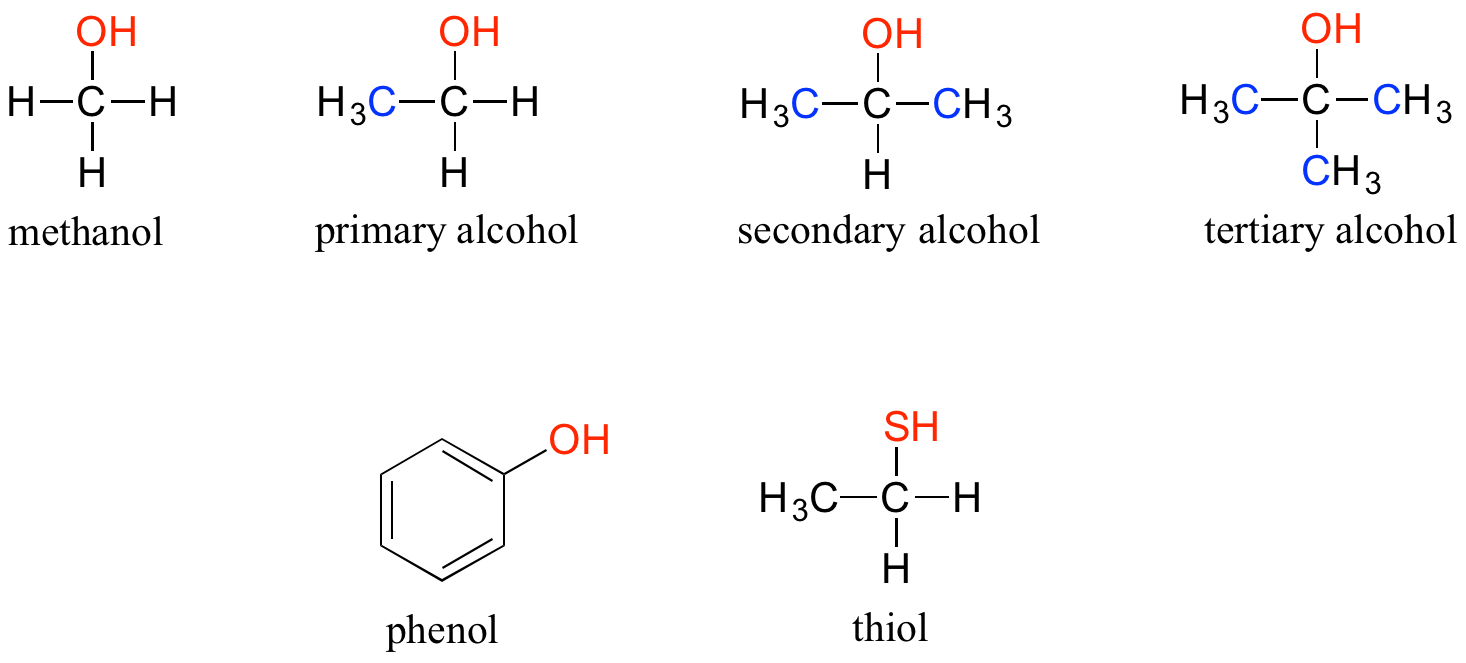
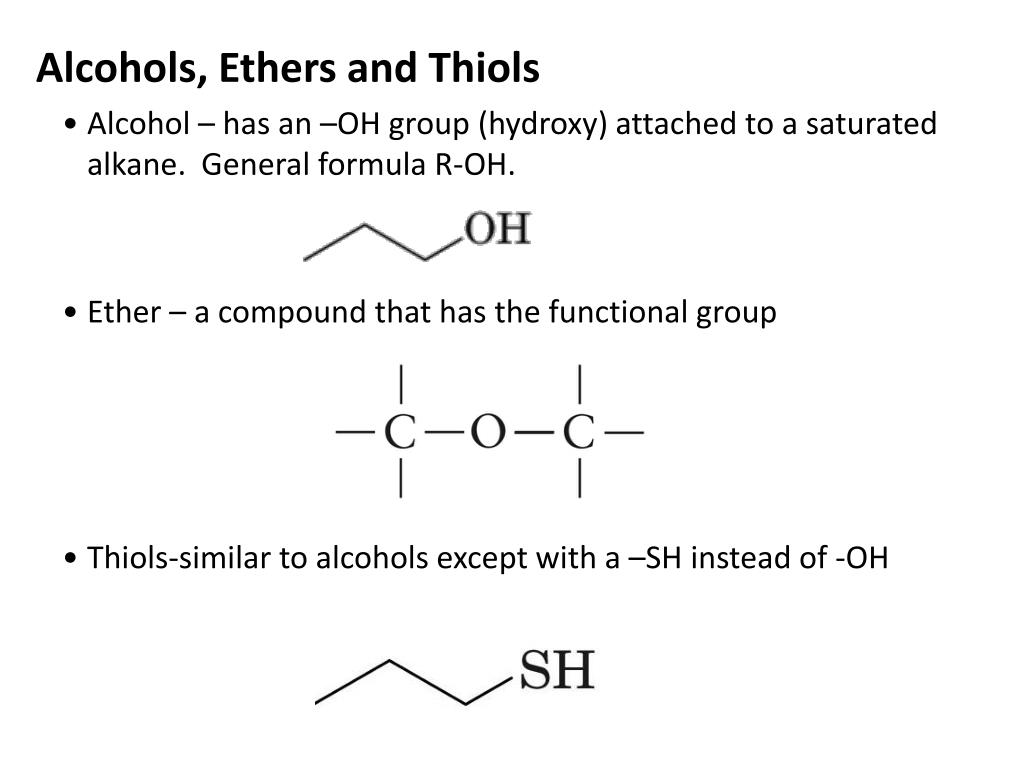
Thiols Have Structures Similar To Alcohols Except That They Contain
Hey there, science explorers! Ever heard of something called a thiol? It sounds a little funny, right? But trust me, these molecules are way cooler than their quirky name suggests. They're like the funky cousins of a very familiar group of chemicals.
You know those handy alcohols, like the ones in hand sanitizer or that nice glass of wine? Thiols have structures that are super similar to alcohols. It's like they're playing a game of "spot the difference" with their molecular blueprints.
But here's where the magic happens. While alcohols have an oxygen atom attached to a carbon chain, thiols swap it out for something a little different. They contain sulfur! Yep, that's right, the same element that makes rotten eggs smell so... distinct.
Now, you might be thinking, "Rotten eggs? That doesn't sound entertaining!" But hold your horses! That sulfur atom is precisely what gives thiols their unique personality. It's the secret ingredient that makes them special and incredibly useful.
Think of it like this: Alcohols are like the polite, well-behaved members of the chemical family. Thiols, on the other hand, are the ones with a bit more flair, a little more oomph. They're the ones who aren't afraid to make a statement.
And what kind of statement do they make? Often, it's a statement of smell. Many thiols have very strong, noticeable odors. Some can be quite pungent, like that infamous sulfur smell we mentioned.
But here's the twist that makes it truly entertaining: not all thiol smells are bad! Some are incredibly desirable. Think of the delicious aroma of a freshly brewed cup of coffee. Believe it or not, thiols are a big part of that wonderful scent!
Or what about the tantalizing fragrance of a ripe grapefruit? Yep, you guessed it – more thiols at play! They contribute to those bright, zesty notes that make citrus so appealing. Who knew a touch of sulfur could be so delightful?

This ability to contribute to complex and pleasant aromas is one of the most captivating aspects of thiols. They're like tiny scent architects, carefully crafting the smells we experience every day. It’s a subtle but powerful influence.
Beyond the fascinating world of aromas, thiols are also incredibly important in many biological processes. They are essential components of certain amino acids, the building blocks of proteins. Without them, our bodies wouldn't function the way they do!
One crucial amino acid that features a thiol group is cysteine. It's like the workhorse of protein structure, helping to form important connections that keep our proteins in shape. These connections are called disulfide bonds, and they're vital for protein stability and function.
Think of proteins as intricate machines. Cysteine, with its thiol group, is like the tiny but mighty bolt that holds crucial parts of that machine together. It’s a small detail with massive implications for life itself.
And it's not just about building blocks. Thiols play roles in how our cells communicate and how our bodies detoxify themselves. They are tiny but mighty chemical messengers and protectors.
In the lab, chemists also find thiols to be incredibly versatile. They are used in the creation of various polymers, the long chains of molecules that make up plastics and other materials. Some of these plastics are even biodegradable!
Thiols are also used in the pharmaceutical industry. They can be found in some medicines, helping to create drugs that treat a variety of conditions. Their unique chemical properties make them valuable for designing effective treatments.
So, while the name "thiol" might sound a bit off-putting, the reality is far more engaging. These sulfur-containing compounds are hidden heroes in our everyday lives, influencing everything from the smell of our morning coffee to the very structure of our bodies.
The "entertainment" in thiols comes from this unexpected duality. They can be associated with strong, sometimes unpleasant odors, yet they are also responsible for some of the most delightful scents we encounter. It's a fascinating contrast.
Their similarity to alcohols is a great starting point for understanding them. It makes them feel familiar, like an old friend with a surprising new hobby. You already know the basic layout; now you just get to explore the exciting differences.
The presence of sulfur is the key. It's this one small atomic swap that unlocks a whole world of unique chemical behaviors. It's a testament to how tiny changes in molecular structure can lead to vastly different properties.

Imagine a chef meticulously adding a tiny pinch of a special spice. That spice might have a strong individual aroma, but in the right context, it transforms the entire dish into something extraordinary. Thiols are like those culinary spices for the chemical world.
Their role in natural flavors is particularly captivating. It shows how nature uses these molecules to create the sensory experiences we enjoy. It’s a reminder that the science behind our favorite tastes and smells is often quite sophisticated.
And the biological significance? That's where thiols really shine as unsung heroes. The fact that they are fundamental to life itself is profoundly interesting. They are silent, essential workers in the grand machinery of biology.
Consider the intricate dance of life happening at the molecular level. Thiols are vital dancers in that performance. They are involved in processes that keep us alive and healthy, often without us ever realizing it.
The versatility of thiols in chemistry is another reason to be intrigued. From creating new materials to developing life-saving drugs, their applications are diverse and impactful. They are building blocks for innovation.
So, the next time you encounter something with a strong, distinctive smell, or enjoy the aroma of your favorite food or drink, take a moment to think about thiols. They might just be the unsung heroes behind the scenes.
Their structures are similar to alcohols, but they contain sulfur. This simple difference is what makes them so special. It's this subtle alteration that leads to such a wide range of properties and functions.
It's the element of surprise that makes thiols so entertaining. Who would have thought that a molecule related to something that smells like a skunk could also be responsible for the delicious aroma of coffee or the vital functions of our bodies?
They are a perfect example of how fascinating chemistry can be when you look beyond the surface. It's a world of tiny particles with immense power and influence.
If you’re curious, a quick search for "thiols in coffee" or "cysteine and disulfide bonds" will open up even more amazing facts. You might just find yourself hooked on these sulfur-containing marvels.
So, next time you hear the word thiol, don't just think of a funny name. Think of complexity, of aroma, of life itself. Think of the entertaining, sulfur-powered cousins of alcohols that make our world a more vibrant and functional place.
They are proof that sometimes, the most extraordinary things come in the most unexpected packages. And their story is far from over; scientists are still discovering new and exciting things they can do!
