The Water Molecule Has A Dipole With The Negative Portion
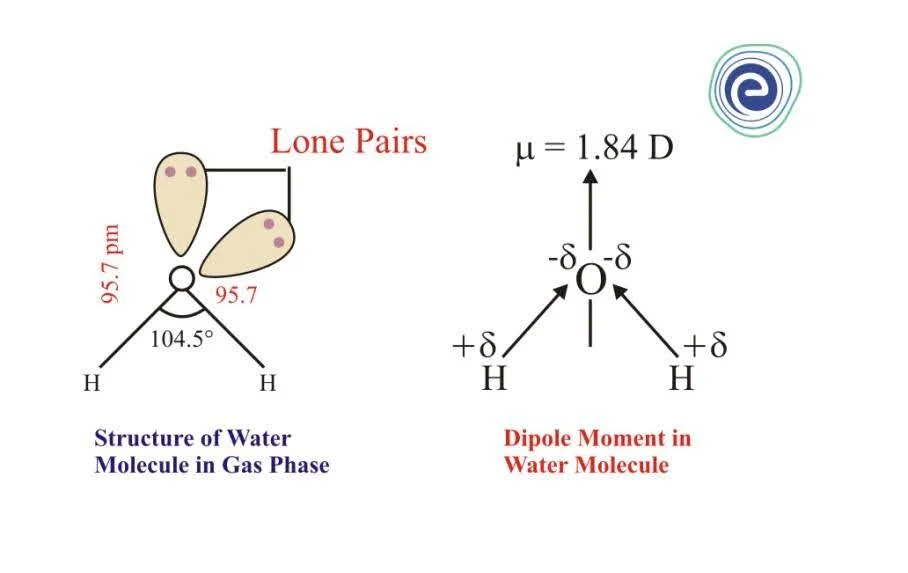
Hey there, fellow curious minds! Ever stop to think about the tiny, tiny things that make up everything around us? We’re talking about molecules, the building blocks of, well, everything. And today, I want to chat about one of the most important and, dare I say, coolest molecules out there: the water molecule. You know, the stuff we drink, swim in, and use to make that perfect cup of tea?
Now, you might be thinking, "Water? What's so fascinating about water?" And I get it. It’s so common, we barely give it a second glance. But trust me, this humble H₂O has some seriously mind-blowing properties, all thanks to a little something called a dipole. Sounds fancy, right? But it’s actually pretty straightforward, and understanding it is like unlocking a secret superpower for water.
So, what exactly is a dipole? Imagine you have a tiny bar magnet. It has a positive end and a negative end, right? Well, some molecules are a bit like that. They’re not perfectly balanced in terms of their electrical charge. They have a slight positive side and a slight negative side. And that’s what we call a dipole.
Now, let's bring it back to our star player: the water molecule. A water molecule is made up of one oxygen atom and two hydrogen atoms. Think of them like little buddies holding hands. The oxygen atom is a bit of a diva. It’s bigger and, more importantly, it’s really, really good at hogging the electrons. Electrons are those tiny, negatively charged particles that zip around atoms. The oxygen atom has a stronger pull on them than the hydrogen atoms do.
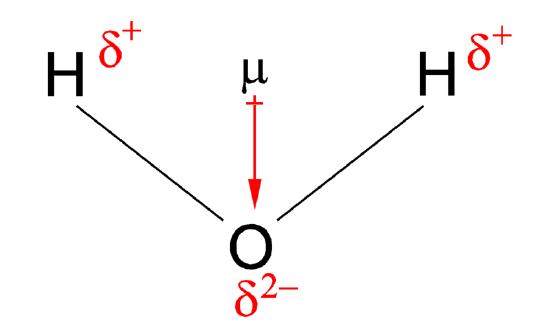
Because the oxygen atom is so good at attracting electrons, it ends up with a slightly more negative charge. It’s like it’s wearing a slightly darker shade of electricity. And the hydrogen atoms, well, they’re left feeling a bit electron-deprived, giving them a slight positive charge. So, in our water molecule, we have an oxygen end that's a little bit negative, and two hydrogen ends that are a little bit positive.

See? It’s got that magnetic-like quality! It’s not a full-blown positive and negative like a giant battery, but a gentle, uneven distribution of charge. This is the heart of why water is so special. This dipole nature is the secret sauce.
Why is this "dipole thing" such a big deal?
Well, it’s like having a bunch of tiny magnets all wanting to connect. Because the water molecule has a negative end and a positive end, these molecules are naturally attracted to each other. The positive hydrogen end of one water molecule will be attracted to the negative oxygen end of another water molecule. It’s like they’re constantly giving each other little nudges and hugs!
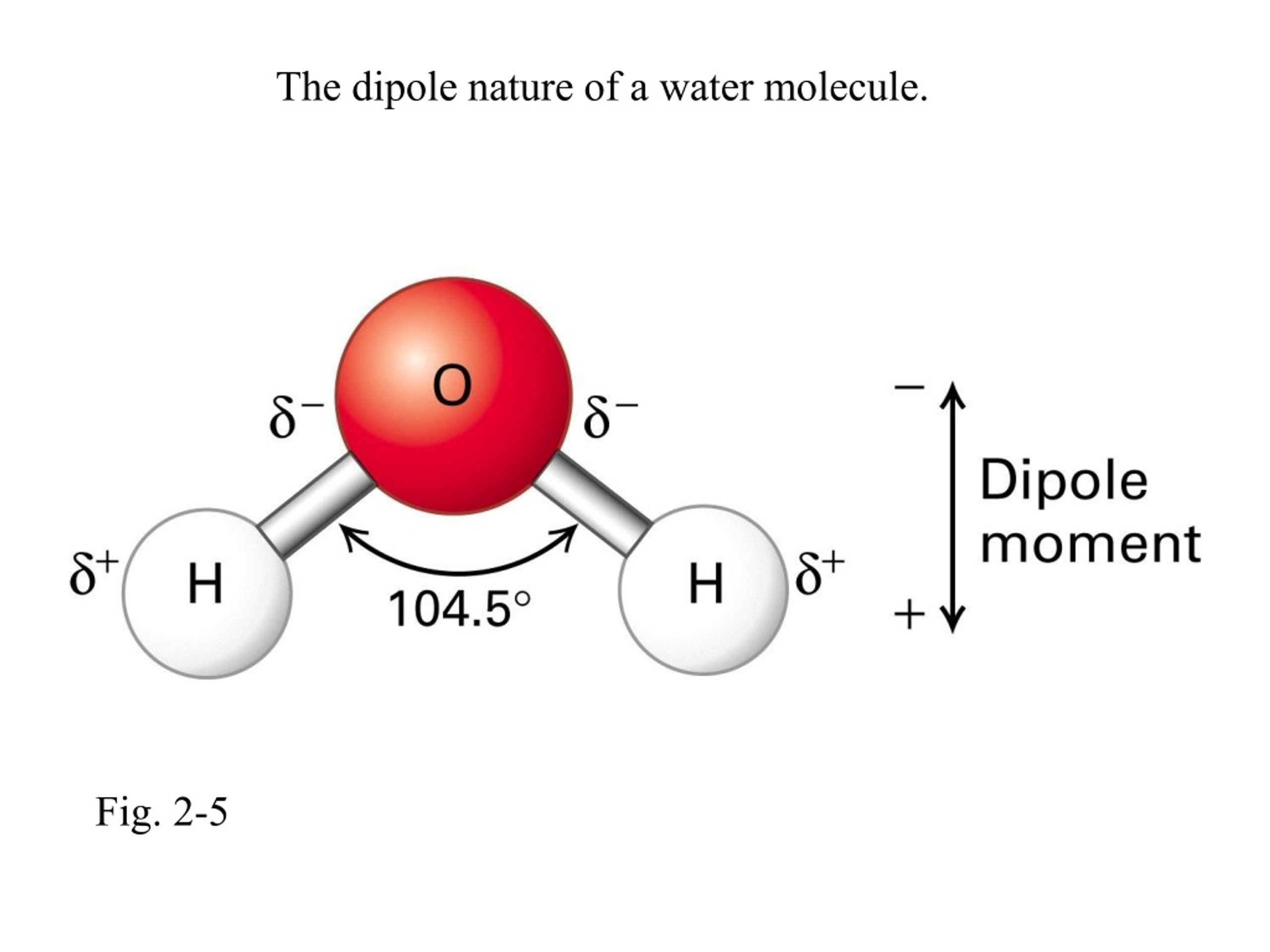
This attraction is called a hydrogen bond. And it’s not just a fleeting connection; these hydrogen bonds are what give water so many of its unique and essential properties. Think about it: water is a liquid at room temperature, which is pretty unusual for a molecule of its size. Many similar-sized molecules are gases! That’s thanks to these persistent little hydrogen bonds holding them together.
Imagine trying to pull apart a bunch of tiny, sticky hands. It takes a bit more effort than just pushing around a bunch of loose marbles, right? Those sticky hands are our hydrogen bonds, keeping the water molecules close. This is why water has a relatively high boiling point and freezing point. It takes a good amount of energy to break enough of those bonds to turn water into steam or to lock it all up into ice.

But it’s not just about staying a liquid. This dipole business also makes water an amazing solvent. What does that mean? It means water is really good at dissolving other substances. Think about dissolving sugar in your tea, or salt in your pasta water. That happens because the slightly positive hydrogen parts of water molecules can surround and pull apart the negative parts of other molecules, and the slightly negative oxygen parts can do the same for the positive parts. It’s like water is giving all these other molecules a friendly hug and helping them to disperse.
So, instead of just floating around in clumps, these other molecules get nicely spread out within the water. This ability to dissolve so many things is crucial for life. Our bodies are mostly water, and all sorts of important chemical reactions happen because our cells can dissolve and transport nutrients and remove waste. It’s like water is the ultimate delivery service and cleanup crew for our internal systems!
And let’s not forget about surface tension. Ever seen a tiny bug walk on water? Or noticed how water forms little droplets on a leaf? That’s surface tension at play, and it’s another direct result of those hydrogen bonds. At the surface of the water, the water molecules are all pulling on each other, creating a sort of “skin” on top. It’s like they’re all holding hands so tightly that they form a flexible, but strong, barrier. It’s pretty neat to think about, isn't it? A bunch of tiny, charged molecules creating a surface strong enough for a water strider!
So, next time you’re drinking a glass of water, or taking a shower, or just looking at a puddle, take a moment to appreciate the incredible science happening at the molecular level. That simple, everyday water molecule, with its slightly negative oxygen and slightly positive hydrogens, is the unsung hero behind so much of what we experience and rely on every single day. It’s a gentle reminder that even the most common things can hold extraordinary secrets, just waiting for us to be a little bit curious.
It’s really quite remarkable, isn’t it? The universe is just packed with these fascinating little details. And the water molecule, with its humble dipole, is a perfect example of how a tiny, invisible property can have such a massive impact on our world. Pretty cool, huh?
