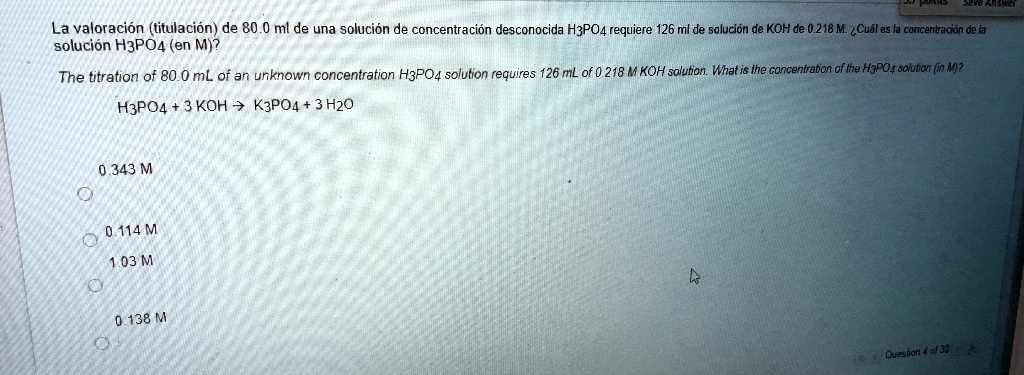
The Titration Of 80.0 Ml Of An Unknown Concentration H3po4
Ever wonder how scientists figure out the exact strength of a mysterious liquid? It’s a bit like a chemical detective story, and today we’re diving into a particularly fascinating case: the
Why is This Chemical Detective Work So Cool?
The beauty of titration lies in its ability to reveal hidden information with remarkable accuracy. Imagine having a potion of unknown power. Titration is the method that tells you exactly how potent it is, down to the last drop. This isn't just for show; it's incredibly useful! Think about the quality control in manufacturing everything from your morning coffee creamer to life-saving medications. Titration ensures they meet specific standards. In environmental science, it helps us monitor the acidity of rain or the purity of water sources. Even in your own kitchen, you might be performing a type of titration without realizing it when you’re baking and measuring ingredients precisely – it’s all about getting the right balance!
The Mission: Unraveling the Acid's Secret Strength
Our specific mission today is to determine the concentration of phosphoric acid (H3PO4). Phosphoric acid is a versatile chemical, found in everything from food additives to fertilizers. Knowing its exact concentration is vital for many applications. For instance, in the food industry, it’s used to add a tangy flavor to beverages and jams, but too much can be unpleasant or even harmful. In industrial processes, precise concentrations are needed for efficient reactions. Without this knowledge, processes could be inefficient, products could be substandard, or safety concerns could arise. The purpose of this titration is to pinpoint exactly how much H3PO4 is dissolved in our 80.0 mL sample.
The benefits of mastering this technique extend far beyond a single experiment. It’s a foundational skill for chemists, biologists, environmental scientists, and even food technologists. It teaches patience, meticulousness, and the importance of observation. Plus, there’s a real sense of accomplishment when you successfully complete a titration and arrive at a precise numerical answer. It’s a tangible result of careful scientific work, proving that even complex problems can be solved with the right tools and a systematic approach.
The Dance of the Dropper and the Flask
So, how do we embark on this detective adventure? We use a technique called titration. Imagine you have your unknown phosphoric acid solution in a flask. We're going to add a solution of known concentration, called a titrant, very carefully, drop by drop, using a specialized instrument called a burette. The titrant in this case is typically a strong base, like sodium hydroxide (NaOH). As we add the NaOH, it reacts with the H3PO4. Phosphoric acid is a triprotic acid, meaning it can donate three protons, so it has multiple reaction stages with the base. This is where it gets interesting! We need a way to know when the reaction is just right – when all the acid has reacted with the base. This magical moment is called the equivalence point.
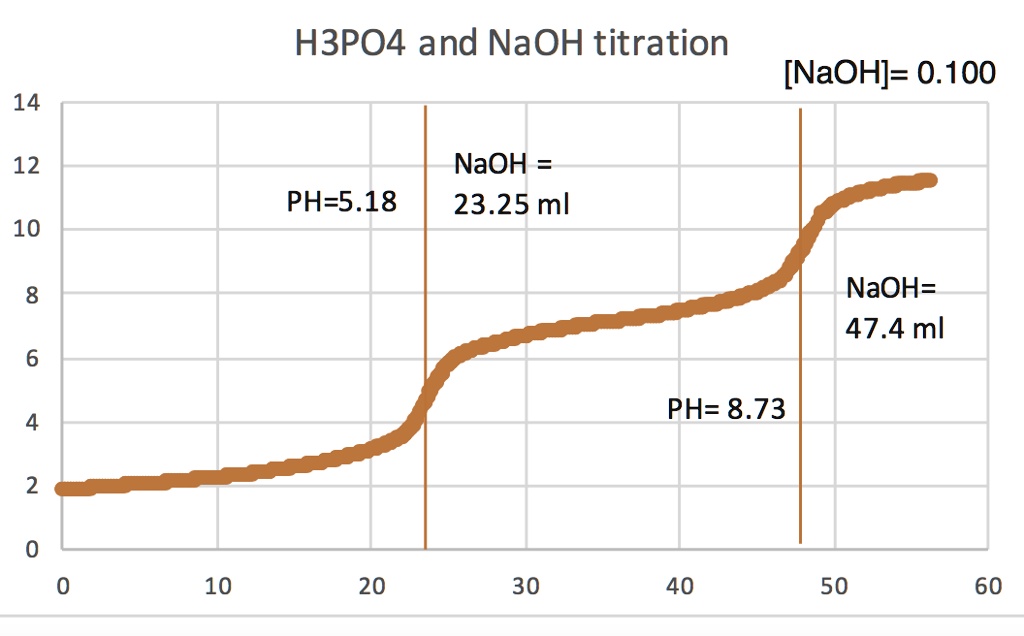
To signal this point, we often use a special chemical called an indicator. Think of the indicator as a tiny flag that changes color when the conditions in the flask change just enough. For the titration of phosphoric acid, common indicators like phenolphthalein might be used, which changes color from colorless to pink (or sometimes a different color depending on the pH range). We’re looking for that point where the color just barely and permanently changes. This is called the endpoint, and it should be very close to our equivalence point.
By carefully measuring how much of the known titrant (the NaOH) we had to add to reach this color change, and knowing the concentration of our titrant, we can work backward. We use stoichiometry – the science of chemical reactions and their proportions – to calculate the concentration of our original phosphoric acid sample. It's a bit like solving a puzzle: we know the size of one piece (the titrant added), and we know how it fits with the unknown piece (the phosphoric acid), so we can deduce the size of the unknown piece. This process ensures we’re not just guessing, but arriving at a scientifically sound and accurate answer for the concentration of our 80.0 mL of H3PO4.
