The Titration Of 25.0 Ml Of An Unknown Concentration H2so4
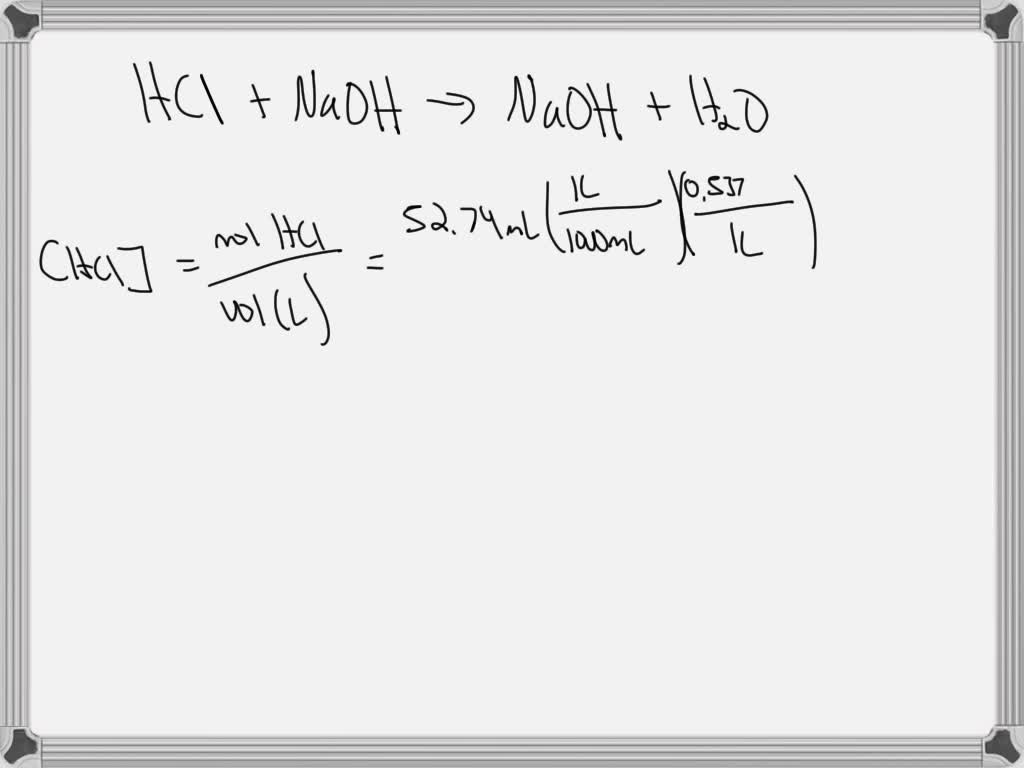
Ever wondered how scientists figure out the exact strength of a liquid without just tasting it (definitely not recommended!)? It's a bit like detective work, but with beakers and chemicals. Today, we're going to peek into the fascinating world of titration, specifically looking at how we might determine the concentration of an unknown sulfuric acid solution. It might sound like complex chemistry, but at its heart, it’s a clever way to measure and understand the world around us.
So, what exactly is this "titration" we're talking about? Think of it as a carefully controlled chemical reaction where we add a solution of known concentration (called a titrant) to a solution of unknown concentration (the analyte) until the reaction is just complete. For our 25.0 mL of unknown sulfuric acid (H₂SO₄), we'd be adding a base, like sodium hydroxide (NaOH), of a precisely known concentration. The magic happens when we reach the equivalence point – the exact moment when the acid and base have completely neutralized each other. We typically signal this point using a special chemical indicator that changes color, giving us a visual cue.
Why bother with all this? The purpose of titration is fundamental: to accurately determine the concentration of a substance. This information is invaluable. It allows us to quantify how much of something is present, which is crucial for quality control, research, and development across countless fields. Think about the benefits: ensuring the correct dosage of medication, checking the acidity of food and beverages, or even analyzing environmental samples for pollutants.
Titration isn't just confined to sterile labs. In education, it's a cornerstone experiment that teaches students about stoichiometry (the quantitative relationships between reactants and products), chemical reactions, and precise measurement. In daily life, the principles are at play when your dentist checks the fluoride concentration in your toothpaste or when food scientists ensure that bottled lemon juice has the expected tartness. Even the manufacturing of fertilizers relies on titration to control nutrient levels.

Curious to explore this a bit further? While performing a full titration at home requires specific safety precautions and materials, you can get a feel for the underlying concept. Imagine you have a glass of lemonade that's a little too tart. You could gradually add a known amount of simple syrup (like a sugar solution of known concentration) until the taste is just right. You're essentially performing a rudimentary form of titration to find the "neutralizing" point for your taste buds! For a more hands-on feel, look for introductory chemistry kits online that might include basic titration exercises. Always remember, though, that working with chemicals, even common ones, requires proper safety guidelines and adult supervision.
The titration of our unknown sulfuric acid is a perfect example of how chemistry allows us to unlock the secrets of concentration and reactivity, making it a truly fascinating and useful endeavor!
