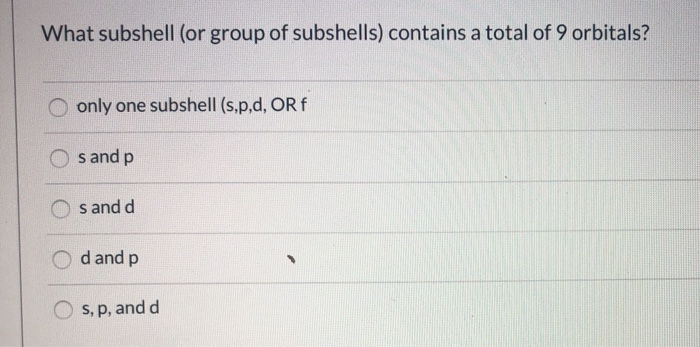
The ________ Subshell Contains Only One Orbital.

Hey there, science explorers! Ever wondered what’s going on inside atoms? It's like a tiny, bustling city, and we’re about to peek into one of its most exclusive neighborhoods. Get ready, because we're talking about the subshell that's super minimalist. Seriously, it’s got the leanest setup of them all.
So, what's this mystery subshell? Drumroll, please... it’s the s subshell! Yep, just ‘s’. Sounds a bit like a whispered secret, doesn’t it?
The Lonely Orbitals of the 's'
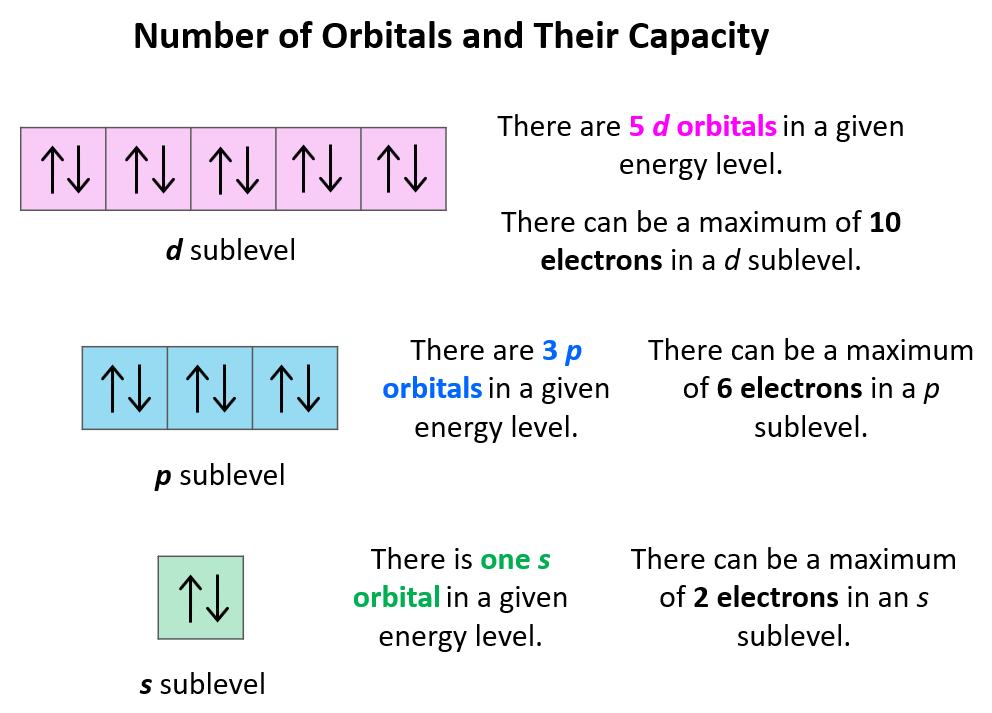
Now, let's talk orbitals. Think of orbitals as the apartments within the atom’s city. Each apartment can hold a maximum of two electrons. And here's the kicker: the s subshell only has one apartment. Just one. That’s it. Talk about a studio apartment of the atomic world!
It’s not like the other subshells, which are like sprawling mansions with multiple rooms. The ‘s’ subshell is more like a chic, minimalist loft. Efficient, right? No wasted space here!
Why Just One Orbital? It's All About Shape!
Why so minimalist? Well, it all comes down to the shape of these electron clouds. The orbitals in an atom aren’t some random blobs. They have specific, mathematically defined shapes. And the ‘s’ orbital has the simplest shape of all: it's spherical. Imagine a perfectly round ball, like a tiny, fuzzy planet. That’s the ‘s’ orbital.
Because it’s so symmetrical and perfectly round, it only needs one orientation in space. No matter how you spin a sphere, it looks the same. It doesn’t have ‘lobes’ pointing in different directions like some of the more flamboyant orbitals we’ll get to later (looking at you, ‘p’ and ‘d’!).
So, this single spherical orbital is where the electrons in the ‘s’ subshell hang out. They’re like two little roommates chilling in their perfectly round, cozy pad.
The 's' Subshell: First Come, First Served
Here’s another fun fact: the ‘s’ subshell is always the first one to get filled in any energy level. It’s like the VIP lounge at the atom’s party. When electrons are filling up the available spots, they hit the ‘s’ subshell first. It’s the most accessible energy-wise.
Think of it like this: the nucleus is the center of the city. The energy levels are like the different districts radiating outwards. The ‘s’ orbitals are in the closest district. So, naturally, electrons pop in there first because it’s the easiest place to be.
This means every single energy level, from the very first one (n=1) all the way up, starts with an ‘s’ subshell. Even when you get to super high energy levels, there’s still that humble, spherical ‘s’ orbital waiting there.
The '1s' Orbital: The OG of Orbitals
Let’s talk about the most famous ‘s’ orbital of them all: the 1s orbital. This is the very first orbital that exists in any atom. It's the electron’s first home, the foundation of atomic structure.

It’s incredibly stable and fundamental. Without the 1s orbital, life as we know it would be… well, impossible. It’s that crucial! All the other orbitals build upon this basic spherical concept.
The 1s orbital is occupied by the two electrons in hydrogen, the simplest atom. And in helium, it’s still just these two electrons, but they're a bit more ‘crowded’ in there. It's like a tiny, two-person dance floor!
What About Other 's' Orbitals?
So, if the ‘s’ subshell always has one orbital, how do we get more electrons in higher energy levels? Ah, this is where it gets even cooler. It’s not that we get more orbitals in the same ‘s’ subshell. Instead, each new energy level gets its own ‘s’ subshell, which also contains just one spherical orbital!
So, energy level 1 has the 1s subshell (with its one spherical orbital). Energy level 2 has the 2s subshell (with its own one spherical orbital). Energy level 3 has the 3s subshell (again, with its own one spherical orbital), and so on. They’re like identical twins, but in different neighborhoods!
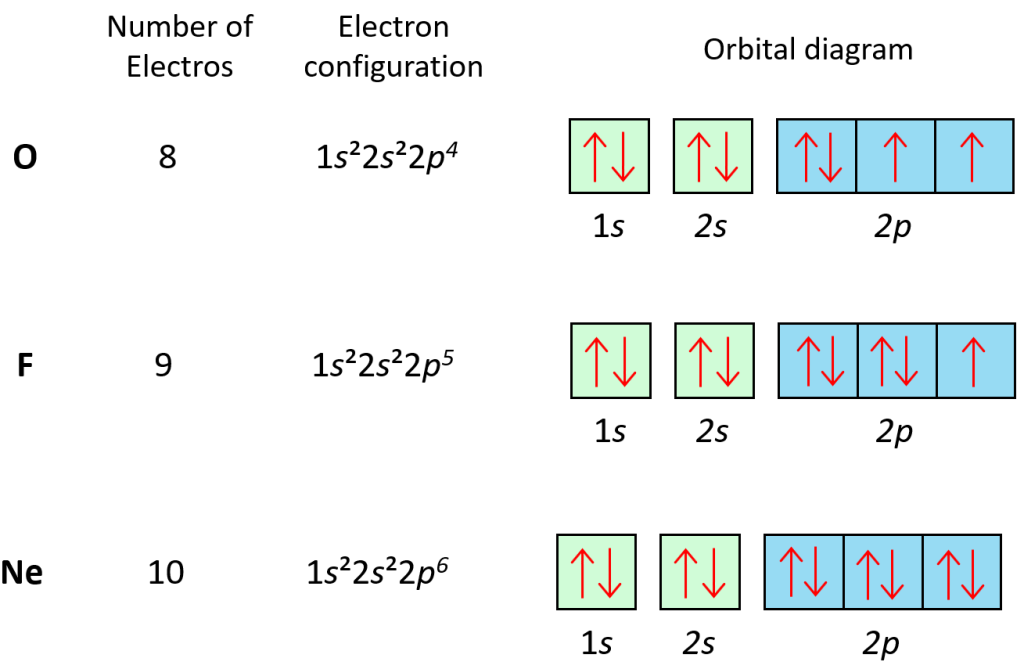
The only difference between, say, the 1s and the 2s orbital is their size. The 2s orbital is a bigger sphere than the 1s orbital. It’s like going from a tennis ball to a basketball. Same shape, just more… spacey.
The 's' Orbital's Big Impact
Even though the ‘s’ orbital is simple and lonely, it plays a HUGE role in chemistry. Many chemical reactions involve electrons in the outermost ‘s’ orbitals. These are the electrons that are involved in bonding and forming molecules. So, this little spherical guy is a big deal in how atoms interact!
Think about how water is formed, or how the carbon in your body came to be. The ‘s’ orbitals are silently working behind the scenes, making all of that happen. Pretty wild, right?
The Funniest Part? It's So Simple!
What makes the ‘s’ subshell so fun to talk about is its sheer simplicity. In a universe of complex electron configurations and tricky quantum numbers, the ‘s’ subshell is like a breath of fresh air. It’s the foundation, the starting point.
It’s like finding out your favorite complicated dish is made with just a few, perfectly chosen ingredients. The ‘s’ orbital proves that sometimes, the most elegant solutions are the simplest ones.
It’s a reminder that even in the unimaginably complex world of atoms, there are these foundational, elegantly simple components that make everything else possible.
So, Next Time You Think About Atoms...
Remember the humble ‘s’ subshell. The one with the single, spherical orbital. The one that’s always first in line. It might not have the flashiest shapes or the most rooms, but it’s absolutely essential. It’s the bedrock of atomic structure, the quiet achiever of the subatomic world.
And that, my friends, is a pretty cool thing to know. Science can be all about the grand mysteries, but it’s also about appreciating the fundamental elegance of things. The ‘s’ subshell? It’s got that in spades. Keep exploring!
