The Reagent Needed To Convert 2-butyne To Trans-2-butene Is

Hey there, fellow explorers of the wonderfully weird world of chemistry! Ever find yourself staring at a tube of 2-butyne, that nifty little alkyne, and wondering, "How do I get this sassy triple bond to chill out and become a smooth, trans-focused double bond?" If your answer is a resounding "Heck yeah, I do!" or even a more hesitant "Uh, what's an alkyne?", then grab your favorite mug of artisanal coffee (or maybe a classic can of Coca-Cola, your call!), settle in, and let's chat about a bit of chemical transformation that's as satisfying as finding a perfectly ripe avocado.
We're talking about the magical journey from 2-butyne to trans-2-butene. It’s like taking a high-energy rock star and coaxing them into a sophisticated jazz ensemble. Both have energy, but the vibe is totally different. And just like you wouldn't send a mosh pit invitation to a chamber music concert, you need the right tool for this specific molecular makeover.
The Star Player: Dissolving Metal Reduction
So, what's the secret sauce? What’s the reagent that can tame that potent triple bond in 2-butyne and guide it towards a neat, tidy, and crucially, trans configuration in 2-butene? Drumroll, please... it’s the classic, the tried-and-true, the absolute legend in its own right: dissolving metal reduction.
Think of it this way: you have your 2-butyne, all coiled up with its three bonds, ready to party. We want to selectively break one of those bonds and introduce hydrogen atoms in a way that they end up on opposite sides of the new double bond. This is where the magic of dissolving metal reduction really shines. It’s a controlled process, like a skilled chef carefully deglazing a pan.
Unpacking the "Dissolving Metal" Vibe
What does "dissolving metal" even mean? It’s not like you’re chucking your car keys into a beaker! In the lab, this typically involves an alkali metal – think sodium (Na) or lithium (Li) – dissolved in a liquid ammonia (NH3) solution. Yes, liquid ammonia! It sounds a bit intense, like something out of a sci-fi movie, but it’s actually a pretty common and effective solvent in organic chemistry.
The alkali metal, when dissolved in ammonia, donates an electron. This creates a solvated electron, which is basically an electron surrounded by ammonia molecules. These are super reactive little critters. It's like unleashing a fleet of tiny, electron-wielding delivery drones.
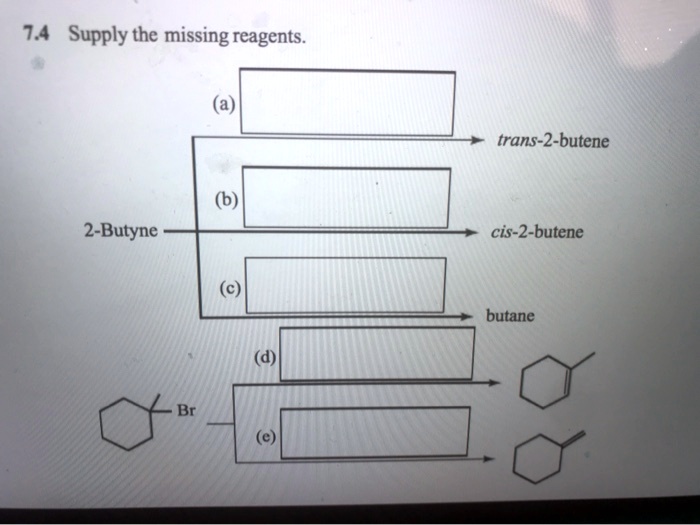
When our 2-butyne molecule encounters these electron-rich environments, a series of fascinating events unfold. The electrons from the dissolving metal attack the triple bond, initiating a reduction process. This reduction happens step-by-step, and the beauty of this method is that it strongly favors the formation of the trans isomer of 2-butene.
Why trans and not cis? Great question! The mechanism involves the formation of a vinyl radical anion, which then abstracts a proton from the ammonia. The subsequent steps, including further electron transfer and protonation, are geared towards producing the more thermodynamically stable trans isomer. It’s like nature’s preference for a more spread-out, less crowded arrangement. Think of it as the molecular equivalent of personal space, which, let’s be honest, we all appreciate.
Why Not Other Reducing Agents? The Plot Thickens
Now, you might be thinking, "Can't I just use, like, a regular hydrogen gas and a catalyst?" And the answer is, well, yes, you can reduce alkynes with hydrogen gas and a catalyst like palladium or platinum. That’s called catalytic hydrogenation. But here's the catch: standard catalytic hydrogenation, with a catalyst like Pd/C, tends to be a bit too enthusiastic. It’ll happily reduce the alkyne all the way to an alkane (in this case, butane), or if you stop it mid-way, it often favors the cis isomer.

Imagine you’re trying to turn down the volume on a loud stereo. Catalytic hydrogenation with a standard catalyst is like slamming the mute button. You get silence, but you lose all the nuanced sound. Dissolving metal reduction, on the other hand, is more like using a sophisticated volume knob. It’s precise, controlled, and gives you the specific outcome you’re after.
There are also special catalysts, like the Lindlar catalyst (palladium poisoned with lead acetate and quinoline), which do allow for the selective reduction of alkynes to cis alkenes. But for our goal of achieving the trans isomer, Lindlar is the opposite of what we want. It’s like trying to bake a cake by using salt instead of sugar – technically a food ingredient, but the result is… decidedly not what you intended.
Practical Tips for the Aspiring Alkyne Alchemist
So, if you’re ever in a situation where you need to perform this particular alkyne-to-alkene transformation, here are a few things to keep in mind:
- Safety First, Always! Working with alkali metals and liquid ammonia isn't exactly a walk in the park. Alkali metals are highly reactive, especially with water, and liquid ammonia is a gas at room temperature and pressure, so it needs to be handled at low temperatures. Always wear appropriate personal protective equipment (PPE) like gloves, eye protection, and a lab coat. And, of course, ensure you're in a well-ventilated area or fume hood. Think of it as essential gear for your molecular adventure, like wearing a helmet when you’re cycling.
- Keep it Cold! Liquid ammonia boils at a rather chilly -33°C (-27.4°F). So, you’ll likely need a low-temperature bath (like dry ice and acetone) to keep your reaction mixture properly cooled. This isn’t just about comfort; it’s crucial for the stability of the liquid ammonia and the efficacy of the reaction.
- Patience is a Virtue. This reaction, like many in organic chemistry, requires a bit of patience. The electron transfer and protonation steps take time. Don't rush it! Think of it like waiting for sourdough bread to rise – good things come to those who wait.
- The "Quench" is Key. After the reaction has proceeded, you’ll need to "quench" it, usually with a proton source like an ammonium salt (e.g., ammonium chloride). This stops the reaction and neutralizes any residual reactive species. It’s the equivalent of a chef saying, “And now for the final flourish!”
Fun Facts and Cultural Connections
Did you know that the discovery of alkali metals by Sir Humphry Davy in the early 19th century was a watershed moment in chemistry? He used electrolysis, a process of using electricity to drive non-spontaneous chemical reactions, to isolate these fascinating elements. It was like discovering a whole new palette of colors for chemists to paint with!

And liquid ammonia? It’s not just a lab reagent. It’s used in industrial processes and was even a refrigerant before modern CFCs (and then, thankfully, their replacements) came along. It’s got a bit of a reputation for its pungent smell, so maybe don’t try to sniff your ammonia solution like it’s a fine perfume. Stick to the roses for that.
The concept of cis and trans isomers is a fundamental one in organic chemistry. It’s like the difference between two people sitting next to each other on a bench (cis) versus one on each end (trans). In the case of alkenes, the trans configuration is often more stable because the bulky groups attached to the double bond are further apart, minimizing steric hindrance. This principle pops up everywhere, from the structure of DNA to the shapes of drug molecules.
Think about it: the way molecules are arranged can drastically alter their properties and how they interact with the world. It’s a bit like how the arrangement of furniture in a room can make it feel spacious and welcoming or cramped and chaotic.
Beyond the Beaker: A Daily Dose of Molecular Thinking
So, why are we geeking out about this specific chemical reaction? Because at its heart, it’s a story about transformation, control, and achieving a desired outcome through careful selection of tools and methods. It’s a reminder that even seemingly complex processes can be understood and managed with the right knowledge and approach.
In our daily lives, we’re constantly performing our own little "chemical reactions." We take raw ingredients and transform them into a meal. We take scattered ideas and organize them into a coherent plan. We take challenging situations and, with a bit of effort and the right strategy, transform them into learning experiences.
Just like dissolving metal reduction offers a specific way to get a trans alkene from an alkyne, understanding our own processes allows us to steer towards preferred outcomes. It’s about recognizing the tools available to us – our skills, our perspectives, our resources – and applying them thoughtfully. Sometimes, we need a broad-spectrum approach, and other times, like with the 2-butyne transformation, we need that precise, targeted reagent. The key is knowing which one to reach for.
So, the next time you're faced with a "molecular" challenge in your own life, take a moment. What's your "2-butyne"? What's your desired "trans-2-butene"? And more importantly, what’s your "dissolving metal reduction" – what specific, precise tool or approach can you employ to get you there? It's a thought that can bring a bit of elegant chemistry to even the most mundane of days.
