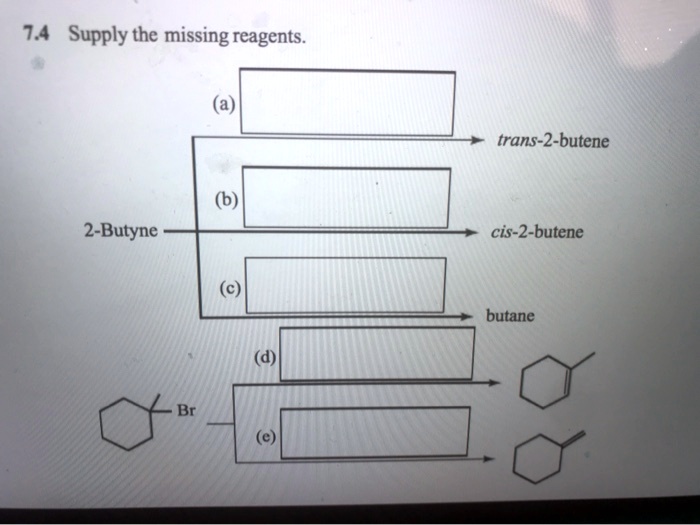
The Reagent Needed To Convert 2-butyne To Cis-2-butene Is

Hey there, science curious friends! Ever feel like chemistry is this big, scary monster locked away in a lab coat? Nah, it's actually way more like a quirky party. And today, we're crashing a super cool one: the transformation of 2-butyne into cis-2-butene. Sounds fancy, right? But trust me, it’s more like a magic trick with a very specific, super-duper important reagent. Think of it as the guest of honor at our chemical bash.
So, what's the big deal about turning this 2-butyne thingy into cis-2-butene? Well, it’s all about how the atoms are arranged, especially those double bonds. 2-butyne has a triple bond. Bam! Triple threat. Cis-2-butene has a double bond, and here's the fun part: the two methyl groups (those are like little carbon arms sticking out) are on the same side of that double bond. It's like they're holding hands. Aww, cute!
But how do we get there? We can't just wave a wand and poof! We need a special guest. A reagent. The magic ingredient. The one and only _________ (drumroll, please!).
The Star of the Show: What's the Reagent?
Drumroll fades. The reagent that turns 2-butyne into cis-2-butene is none other than hydrogen gas (H₂), but with a super important, almost diva-like condition. It can't just be any old hydrogen. Oh no. It needs to be carefully controlled. We're not trying to turn it into something else entirely, like butane (which is just saturated with hydrogens, no more double or triple bonds). We want to stop at the double bond stage, and crucially, we want that cis configuration. It's like asking for a perfectly ripe avocado – you don't want it too green, and you definitely don't want it mushy brown.
So, plain hydrogen gas? Not quite enough to get the job done with the precision we need. We need a helper. A chaperone. Someone to tell the hydrogen, "Whoa there, cowboy! Slow down!"
Enter the Catalyst: The Party Organizer!
This is where things get really interesting. To make sure our hydrogen gas plays nice and gives us exactly what we want – cis-2-butene – we need a special kind of helper called a catalyst. Catalysts are the unsung heroes of chemistry. They speed up reactions or guide them down specific paths without actually getting used up themselves. They’re like the amazing DJ at our party, keeping the energy up and the tunes just right. They don’t dance themselves, but they make the whole dance floor a better place.
For our 2-butyne to cis-2-butene mission, the go-to catalyst is a famously finicky one: Lindlar's catalyst. You heard it right. It’s named after some clever scientist, probably a bit of a perfectionist himself. Lindlar’s catalyst is basically palladium (a shiny metal) that’s been intentionally poisoned. Yep, poisoned! But in a good way. It's like giving a bodyguard a very specific set of instructions: "Only let the dancers do the waltz, and make sure they all face the same direction. No solo pirouettes, and definitely no mosh pits!"
How do we "poison" palladium? We treat it with something like lead acetate or quinoline. These chemicals coat the palladium surface, making it less reactive. This is crucial! It stops the reaction from going too far. Remember that triple bond in 2-butyne? Hydrogen gas loves to break bonds. Without our Lindlar’s catalyst, it would just keep adding hydrogens until we have plain old butane. Boring!
The poisoned palladium surface of Lindlar’s catalyst is just perfect for adding one molecule of hydrogen across the triple bond. And here’s the kicker: the way it presents the molecules to each other forces the incoming hydrogens to add to the same side of the forming double bond. It’s like a tiny, perfectly arranged chemical handshake. This geometry is what gives us the cis isomer. The two methyl groups end up on the same side, just like we wanted!
Why is This Fun to Talk About?
Honestly, it's the precision! It's the idea that you can take something with a triple bond (a whole lot of chemical energy and potential!) and, with the right nudge, guide it to become something with a double bond, but with a specific, cis arrangement. It's like being a master sculptor. You're not just breaking stone; you're carefully chipping away to reveal a specific form.

And Lindlar's catalyst? It’s just so delightfully quirky. You have to deactivate a perfectly good metal to make it work better for a specific job. It’s counterintuitive and cool. It’s like saying, "To get the best performance, we need to hold back a little." It shows that sometimes, less is more, especially in the world of chemical reactions.
Plus, think about it. This process is used in real-world applications! While cis-2-butene might not be something you’d buy at the grocery store, understanding these transformations helps chemists create all sorts of useful materials and medicines. It’s all about controlling how molecules behave, and that’s a superpower, wouldn't you say?
The Chemical Dance Floor
So, let’s recap our chemical party. We've got 2-butyne, our eager triple-bonded molecule. We’ve got hydrogen gas, our energetic guest ready to add itself. But the real star, the one who orchestrates the whole affair, is Lindlar's catalyst. It’s the bouncer and the choreographer all rolled into one. It makes sure the hydrogen adds to the triple bond, but only once, and in a way that creates that specific cis geometry. The lead acetate or quinoline is like the velvet rope, controlling who gets in and how they behave.

Without Lindlar’s catalyst, hydrogen would just barge in and add two molecules of itself, giving us fully saturated butane. It would be a chemical free-for-all! But with Lindlar's, it's a choreographed ballet. It’s a testament to how subtle changes in our chemical environment can lead to dramatically different outcomes.
It’s this level of control that makes chemistry so fascinating. It’s not just about mixing stuff together; it's about understanding the intricate dance of atoms and molecules and learning to lead the dance. And for turning 2-butyne into cis-2-butene, the lead dancer is definitely Lindlar’s catalyst.
So next time you hear about a chemical reaction, don't just think about big, scary equations. Think about the star reagents, the quirky catalysts, and the amazing precision that goes into making molecules do exactly what we want them to. It’s a party, and everyone’s invited, especially the perfectly poisoned palladium!
