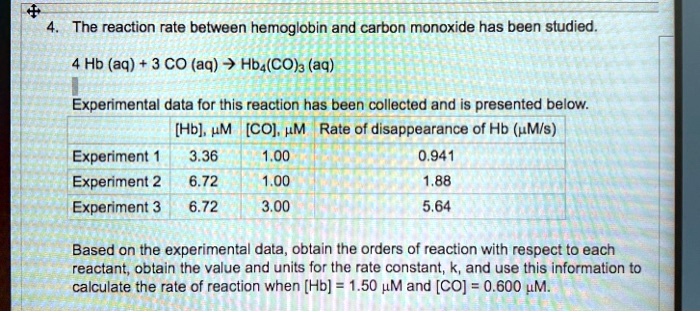
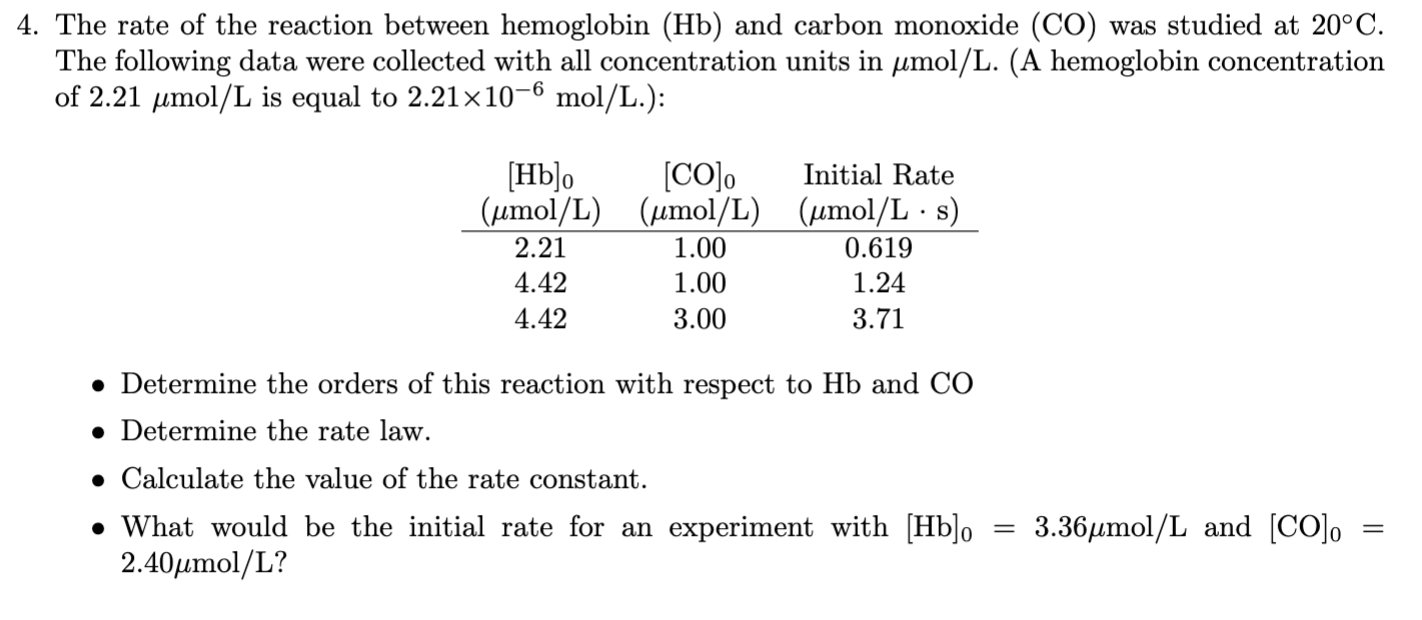
The Rate Of The Reaction Between Hemoglobin And Carbon Monoxide

So, picture this: I'm crammed into a tiny, stuffy car with my family on what was supposed to be a scenic road trip. Think winding mountain roads, picturesque overlooks, the whole nine yards. But the real adventure, as it turns out, wasn't the view. It was the smell. A faint, almost imperceptible, but definitely there smell of exhaust fumes. My dad, bless his mechanically-challenged heart, swore the car was fine. My mom, ever the pragmatist, was already googling “auto mechanic near me.” And me? I was suddenly remembering my high school chemistry class, specifically that one lecture about how some things are just way more dangerous than they seem.
It turns out, that faint exhaust smell was carrying something a little more sinister than just burnt fuel. It was carrying carbon monoxide. And while that trip ended without any drama (phew!), it got me thinking. How does something so seemingly harmless, like a whiff of exhaust, pack such a serious punch? And what’s really going on inside our bodies when we breathe that stuff in?
The Uninvited Guest: Carbon Monoxide
Okay, so let’s dive into the nitty-gritty. Carbon monoxide, or CO as the cool kids call it, is a colorless, odorless gas. Yep, you read that right. Colorless and odorless. That’s its super-power, and also its biggest danger. You can’t see it, you can’t smell it, and you usually don’t feel it coming. It's like the ninja of toxic gases. Pretty sneaky, huh?
It’s produced by incomplete combustion. Think car engines, furnaces, fireplaces, even faulty gas stoves. Basically, anything that’s burning fuel without enough oxygen to do it properly is a potential CO-producing culprit. And that’s where things get interesting, especially for our blood.
Hemoglobin: The Oxygen Delivery Service
Now, let’s talk about our trusty hemoglobin. This amazing protein is like the ultimate delivery driver for our bodies. It’s found in our red blood cells, and its main job is to pick up oxygen in our lungs and ferry it to all the tissues and organs that need it to survive. Pretty crucial role, right? Without hemoglobin doing its thing, our cells would starve for oxygen.
Hemoglobin has a special binding site that’s designed to grab onto oxygen molecules. It’s a really precise fit, like a key in a lock. This binding is reversible, which is important. It picks up oxygen where it's abundant (the lungs) and releases it where it's needed (the body's tissues). Think of it as a well-oiled logistics operation.
The Plot Twist: CO Crashes the Party
Here’s where our uninvited guest, carbon monoxide, comes in. And this is where the chemistry gets a bit wild. It turns out that hemoglobin is way more attracted to carbon monoxide than it is to oxygen. Like, a lot more. We’re talking something like 200 to 250 times more attracted! Can you believe it?
Imagine your hemoglobin is a popular nightclub, and oxygen is a celebrity. CO rolls up, looking all mysterious and smooth, and the bouncer (hemoglobin) basically throws the celebrity (oxygen) out to make room for CO. It’s a hostile takeover, pure and simple.
When CO enters our bloodstream, it finds those precious hemoglobin molecules and, well, it forms a bond. It binds to the same site that oxygen is supposed to bind to. But this bond is much stronger and much harder to break than the oxygen-hemoglobin bond.
So, instead of picking up oxygen, our hemoglobin molecules start picking up carbon monoxide. This creates a compound called carboxyhemoglobin (or COHb). And every time a hemoglobin molecule becomes bound to CO, it’s essentially taken off the oxygen delivery service. It’s gone AWOL, leaving fewer and fewer red blood cells available to carry oxygen.
The "Rate" of Disaster: Why Speed Matters
This is where the concept of the "rate of reaction" becomes super important. It’s not just that CO is dangerous; it’s how quickly it can outcompete oxygen and cause problems. The rate at which hemoglobin binds to CO versus oxygen dictates how fast our bodies become oxygen-deprived.
Several factors influence this rate. One of the biggest is the concentration of carbon monoxide in the air we breathe. If there’s a lot of CO floating around, it’s going to bump into hemoglobin more frequently, increasing the chances of it binding. Think of it like a crowded party versus an empty room. More people (CO) in the room means more interactions.
Another key factor is the partial pressure of oxygen. This refers to how much oxygen is available in the air. If you’re in a room with low oxygen (maybe at a high altitude), even a small amount of CO can have a more significant impact because there’s less oxygen competing for those hemoglobin binding sites in the first place.
And then there’s the affinity itself. As we discussed, hemoglobin’s affinity for CO is just inherently much, much higher than for oxygen. This means that even at lower concentrations, CO can still win the battle for binding sites. It’s like a speed demon vs. a leisurely jogger – CO is just built to move faster and stick around longer.
What Does This Actually Feel Like?
So, what’s the actual physiological impact of this CO takeover? When hemoglobin is bound to CO, our tissues aren’t getting the oxygen they need. This leads to hypoxia, or oxygen deprivation. At first, you might not notice much. Maybe a slight headache, some dizziness, or feeling a bit nauseous. Your body’s trying to tell you something’s up, but it’s subtle.
As the COHb levels increase, the symptoms become more severe. You might experience confusion, shortness of breath, chest pain (especially if you have heart problems), and even loss of consciousness. In extreme cases, it can lead to permanent brain damage or death. And the really scary part is that sometimes people don’t even realize they’re being poisoned until it’s too late. They might just think they’re feeling unwell and go to sleep, never to wake up.
It’s like a slow-acting poison, but one that’s invisible and silent. You don’t get a warning siren. It just creeps in.

The Speed of the Switch: A Chemical Dance
Let's get a little more technical for a moment, because the "rate" is truly the star of this show. The binding of a molecule to another is often described by kinetic parameters, like the rate constants. For hemoglobin and its ligands (the molecules it binds to), there are specific rate constants for binding and dissociation. These constants dictate how quickly a bond forms and how quickly it breaks.
For oxygen, the binding and dissociation are relatively fast. This allows hemoglobin to efficiently pick up and release oxygen throughout the body. For carbon monoxide, the binding rate constant is much higher, and the dissociation rate constant is much lower. This means CO binds very quickly and then hangs on with incredible tenacity.
So, while oxygen is constantly coming and going, CO is like a stubborn guest who’s decided to move in permanently. This imbalance in the binding and dissociation rates is the fundamental reason why CO is so toxic. It’s not just about what it binds to, but how fast and how tightly it binds.
Factors Affecting the Rate of CO Poisoning
Beyond the concentration of CO and the partial pressure of oxygen, there are other things that can influence how quickly someone is affected:
- Activity Level: If you’re exercising, your body needs more oxygen. This means your respiratory rate increases, and you’re breathing in more air. If that air contains CO, you’ll be exposed to it more rapidly, and your hemoglobin will be working harder, making it more susceptible to CO binding.
- Underlying Health Conditions: People with respiratory or cardiovascular issues are often more vulnerable. Their bodies may already be struggling to get enough oxygen, so CO poisoning can hit them harder and faster.
- Duration of Exposure: The longer you’re exposed to CO, the more carboxyhemoglobin will build up in your blood. It’s a cumulative effect. Even low levels of CO can become dangerous over time.
It’s a complex interplay of chemistry and physiology, all happening at a molecular level that has very real, and sometimes tragic, consequences.
![[Solved]: The rate of the reaction between hemoglobin](https://media.cheggcdn.com/study/a68/a689806e-5b4b-4fc2-a8d4-5ef956bf149d/image.jpg)
Preventing the Invisible Threat
Knowing about the rate of reaction between hemoglobin and carbon monoxide isn't just an academic exercise. It’s crucial for understanding how to protect ourselves and our loved ones. The most effective way to prevent CO poisoning is to prevent exposure in the first place.
This means:
- Installing and regularly maintaining carbon monoxide detectors in your home. These are lifesavers, plain and simple. They’re designed to alert you to the presence of CO before it reaches dangerous levels.
- Ensuring that fuel-burning appliances like furnaces, water heaters, and stoves are properly installed and vented.
- Never running a car, generator, or other gasoline-powered engine in an attached garage or enclosed space, even with the door open.
- Using portable generators only outdoors and away from windows and doors.
- Getting your chimney and fireplace inspected and cleaned annually.
It’s about being proactive. That faint smell in the car? It was a subtle warning. We often ignore those subtle warnings because they’re not dramatic. But with carbon monoxide, the lack of drama is exactly what makes it so dangerous.
A Final Thought (and a Reminder!)
The speed at which hemoglobin binds to carbon monoxide, and the strength of that bond, is a fascinating and terrifying aspect of biochemistry. It highlights how something as seemingly simple as breathing can be compromised by an invisible, odorless gas. It’s a stark reminder that even the most fundamental biological processes can be disrupted by chemical interactions.
So, next time you’re thinking about home safety, remember the silent invader. Invest in those CO detectors. Make sure your appliances are running smoothly. Because when it comes to carbon monoxide and your hemoglobin, the race is on, and you want to make sure oxygen wins, every single time. Stay safe out there, folks!
