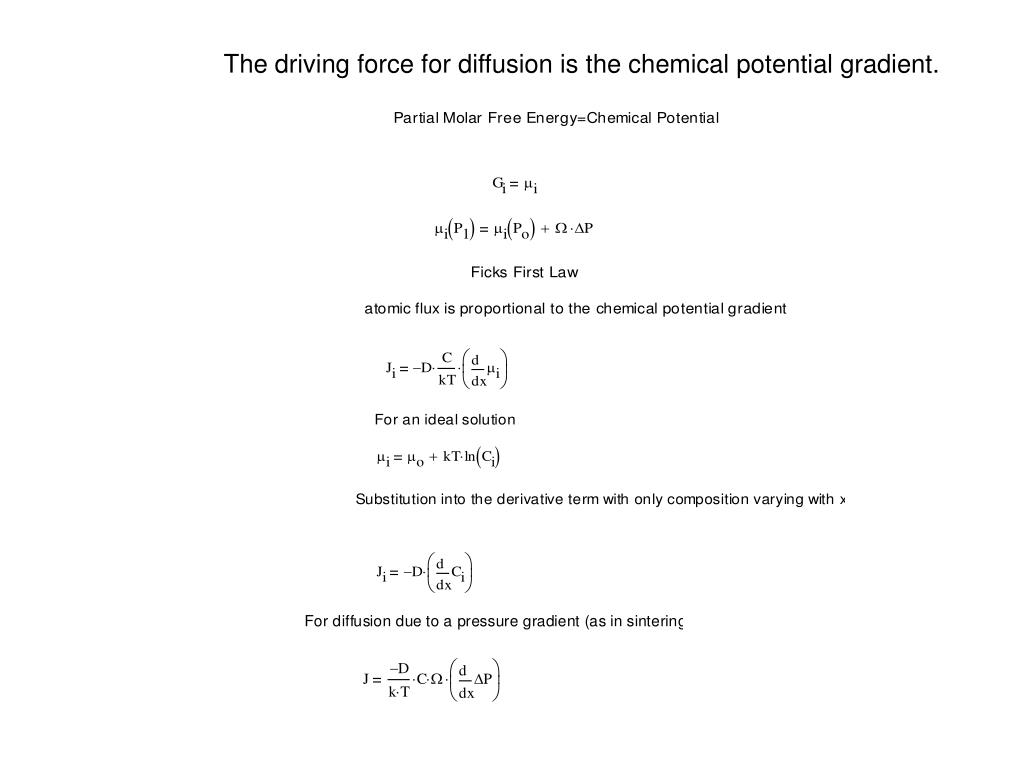
The Principal Force Driving Movement In Diffusion Is The
Hey there, coffee buddy! Grab a refill, would ya? We're gonna chat about something super cool, something that’s happening all around us, all the time. Like, right now, even. It’s this whole idea of diffusion. You know, when stuff just… moves? From here to there? Ever wonder why it does that? It’s not magic, I promise! Although sometimes it feels like it, right?
So, the big kahuna, the main engine, the driving force behind all this movement in diffusion? It’s actually pretty simple once you break it down. It’s not some tiny invisible hand pushing things along, though wouldn’t that be adorable? Nope. It all boils down to one crucial concept: the concentration gradient. Catchy, huh?
Think about it. What does "concentration" even mean in this context? Basically, it’s just how much of something is hanging out in a particular spot. Is there a whole lot of it? High concentration! Is there just a little bit? Low concentration. Easy peasy.
And the "gradient"? That's just the difference between these two amounts. It's like a slope, a hill, a slide! Stuff loves to go downhill, right? Or, in this case, from where there's a lot of something to where there's not so much of it. It’s like when you're at the top of a water slide, and you just know you're gonna go down. Gravity, right? Well, concentration gradient is kind of the molecular version of that gravity.
Imagine you’ve just brewed a super strong cup of coffee. The smell! Oh man. That incredible coffee aroma is everywhere in your kitchen, right? But if you were to somehow trap all those delicious scent particles in just one little corner of the room, what would happen? They wouldn’t just stay there, huddled together like introverts at a party. Nope! They’d start to spread out.
They’d naturally drift, waltz, or even zoom (depending on how energetic they’re feeling!) into the areas where there aren't as many coffee smell particles. It's like they're trying to make friends with the air molecules in the rest of the room, you know? They want to achieve a state of, dare I say it, equilibrium. Balance! Ah, sweet, sweet balance.
So, the coffee smell molecules were at a high concentration right where you brewed it. And the air molecules further away? They had a low concentration of coffee smell. That difference, that gap? That's your concentration gradient. And it’s this very gradient that’s the push.

It’s like having a crowded room and a deserted hallway. People naturally want to move from the packed room to the empty hallway, don't they? It's not because they dislike the room, necessarily. It's just… there’s more space! More freedom! More room to breathe, literally!
And that’s exactly what’s happening at the microscopic level. Those little molecules – whether they’re coffee scent molecules, sugar molecules dissolving in water, or even oxygen in your lungs – they’re all just constantly jiggling and wiggling. They have this inherent kinetic energy. They're never truly still, even if they look like it.
So, when you have a bunch of them all bunched up in one place (high concentration), they’re bumping into each other like bumper cars at the fair. And where do they go when they get bumped? They go in a direction! And over time, if there’s an area with fewer of them, they’ll tend to drift that way.
It’s not a coordinated effort, you see. No one’s sending out little memos: "Okay team, let's all migrate to the less crowded area!" It's pure, unadulterated, random motion. But when you have millions, billions, trillions of these little guys randomly bouncing around, the net effect is this movement from high concentration to low concentration.
Think of a big party. Everyone’s milling about, chatting, dancing, having a grand old time. Then, someone opens a door to a quieter, empty room. A few people might wander in. Then a few more. It's not a stampede, but gradually, the energy and the people will spread out a bit. The party (high concentration) becomes a little less intense, and the empty room (low concentration) gets a little livelier. The gradient is shrinking!
This whole process, this striving for evenness, is pretty fundamental to life, you know? It’s not just about your morning coffee smell. It’s about how your body works! Pretty wild to think about, right?
Let’s get a bit more scientific, but still keep it chill. In biology, we see this all the time. Take oxygen. You breathe in air, which has a nice, high concentration of oxygen. Your lungs? They’re lined with these tiny air sacs called alveoli. And the blood flowing through the capillaries next to these alveoli? It has a lower concentration of oxygen because your body’s cells have been using it up.
So, what happens? Bam! Diffusion kicks in. The oxygen molecules, super happy in the air-filled alveoli, start to move across the thin membranes into the blood. They’re going down their concentration gradient. They’re heading to where they’re needed!
And then, when that oxygen-rich blood gets to your muscles or your brain, what happens? Your cells are chugging along, using up that oxygen, making it a low concentration in the cells themselves. So, the oxygen in the blood? It diffuses out of the blood and into your cells. See? It's a constant dance!
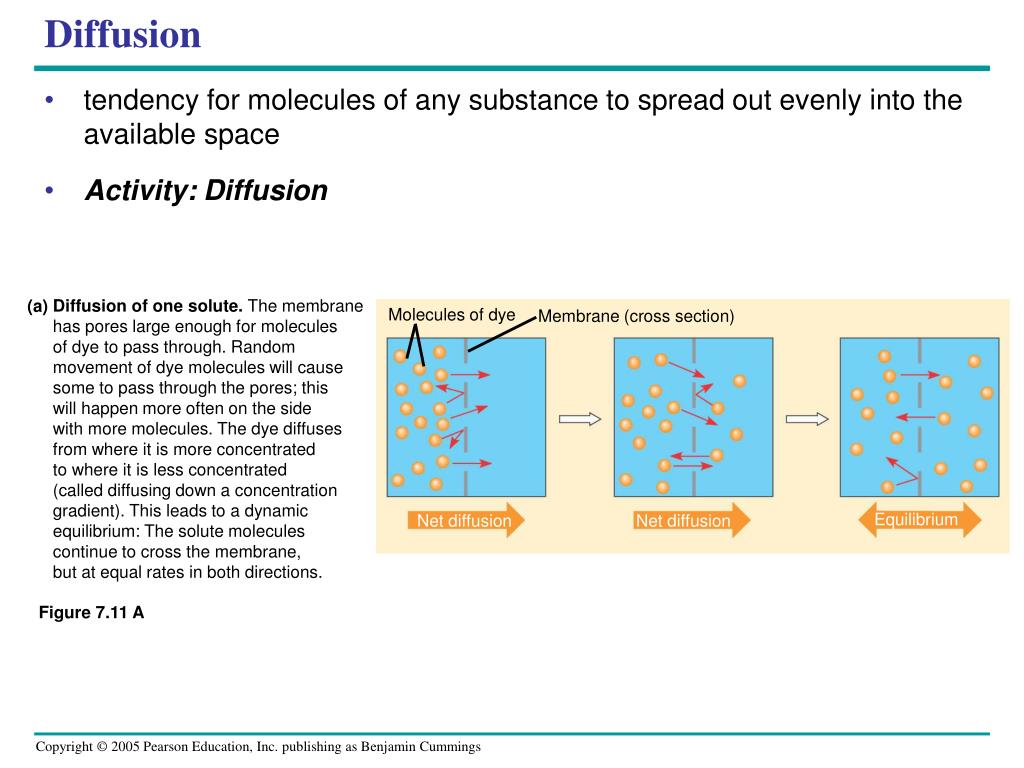
It works the same way with carbon dioxide, that stuff we breathe out. Your cells are constantly producing it as a waste product. So, the concentration of carbon dioxide is high inside your cells and lower in the blood. What do you think happens? You guessed it! Carbon dioxide diffuses from the cells into the blood.
Then, when that blood reaches your lungs, the concentration of carbon dioxide is higher in the blood than in the air you’re about to exhale. So, poof! Carbon dioxide diffuses from the blood into the alveoli, and then you breathe it out. It’s like the ultimate recycling program, powered by diffusion!
Even something as simple as dissolving sugar in your tea relies on this principle. You put a spoonful of sugar in your hot tea. Initially, the sugar is all concentrated at the bottom. The tea molecules are like, "Whoa, what's all this?!" And the sugar molecules are like, "Hey, we’re trying to spread out here!"
Slowly, the sugar molecules start to break apart from the lump and drift through the tea. They’re moving from an area of high sugar concentration (where you dumped it) to areas of low sugar concentration (the rest of the tea). Eventually, your whole cup of tea tastes sweet, all thanks to that gradient.
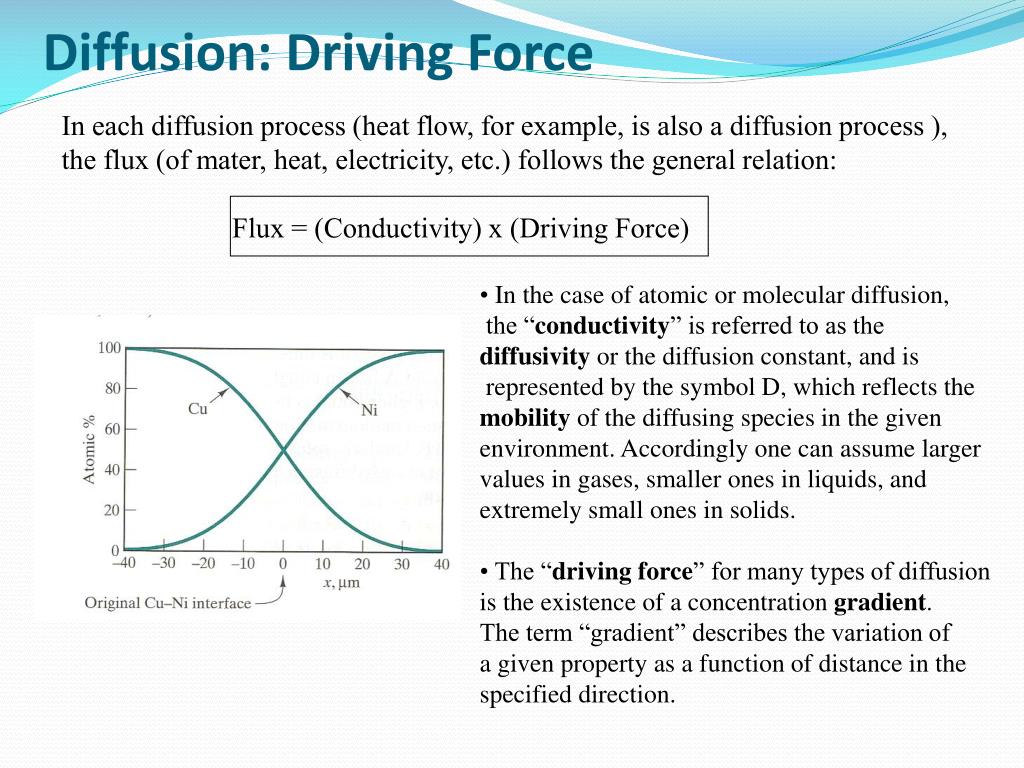
It’s so much more elegant than active transport, where cells have to expend energy to move things against their gradient. Diffusion is the lazy river of molecular movement. It just goes with the flow, from where there’s a lot to where there’s less. No effort required, just the inherent nature of things wanting to spread out.
Now, sometimes things can mess with this natural tendency. If the temperature changes, for instance, molecules get more or less energetic. More energy? More bumping, faster diffusion. Less energy? Slower diffusion. Think about trying to dissolve sugar in iced tea versus hot tea. The hot tea wins, right?
Or what about the distance? If the gap to cross is super short, diffusion happens quickly. If it's a really long haul, it takes more time. That’s why those alveoli in your lungs are so incredibly thin – to make the diffusion distance for oxygen and carbon dioxide as small as possible. Efficiency, my friends!
But the absolute, undeniable, number one reason why things move in diffusion? It's that concentration gradient. It’s the underlying force, the potential energy waiting to be released as molecules spread out. It’s the universe’s way of saying, "Hey, let's chill out and be evenly distributed, shall we?"
So next time you smell something delicious, or watch sugar disappear into your drink, or even just take a deep breath, remember the humble, yet mighty, concentration gradient. It’s the silent hero of molecular movement, the invisible hand that keeps so much of our world, and ourselves, ticking along. Pretty neat, huh? Now, about that refill…?
