The Maximum Number Of Electrons In A Single D-subshell Is
Ever wondered about the secret lives of electrons? These tiny, zippy particles are the building blocks of everything around us. And guess what? They have their own little neighborhoods called subshells! Today, we're diving into a super cool one: the D-subshell.
Think of atoms like tiny cities. Inside these cities are different districts. These districts are called shells. And within each shell, there are even smaller neighborhoods, the subshells. It's like having neighborhoods within neighborhoods!
We've got S, P, D, and F subshells. Each one has a slightly different vibe, a different shape, and a different capacity for holding our electron pals. Today, our spotlight is on the D-subshell. It's got a bit of a reputation for being a bit more complex, but that's what makes it so fascinating!
The Amazing D-Subshell: A Party for Ten!
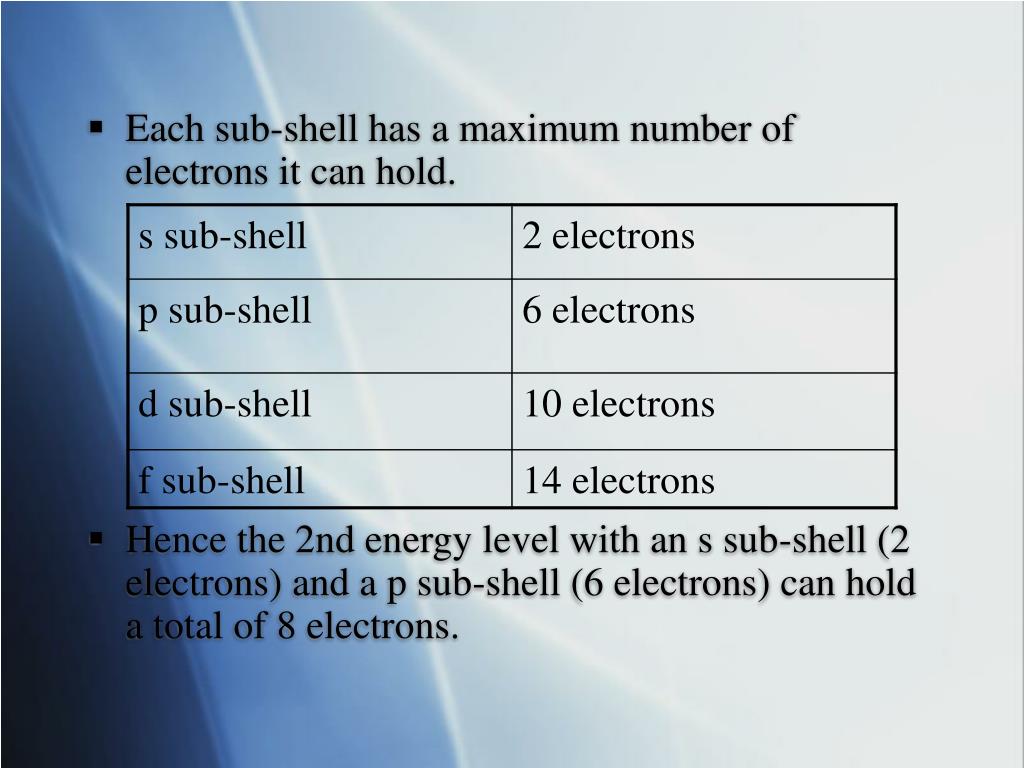
So, what's the big deal about the D-subshell? Well, it's all about how many electrons can squeeze into this particular electronic hangout. And the answer, my friends, is a whopping ten! That's right, the maximum number of electrons that can call a single D-subshell home is ten.
Imagine a party bus that can hold exactly ten people. That's kind of like our D-subshell. It has a perfect capacity, not too crowded, not too empty. It's just right for a group of ten electrons to chill together.
This number, ten, is a fundamental part of how elements behave. It dictates how they bond with other elements and what kinds of cool compounds they can form. It’s like a secret handshake that the D-subshell uses with the rest of the atomic world.
Why Ten is So Special for D-Subshells
You might be asking, "Why ten? Why not eight, or twelve?" That's a great question! It all comes down to the intricate dance of quantum mechanics. Electrons don't just float around randomly; they occupy specific spaces and have specific energies.
The D-subshell is structured in a way that allows for five specific "orbitals." Think of orbitals as individual rooms within the D-subshell neighborhood. Each room can hold a maximum of two electrons. And 5 rooms times 2 electrons per room equals… you guessed it, ten electrons!
+Maximum+Number+of+Electrons.jpg)
These five orbitals have some pretty wild shapes. They aren't simple spheres like in the S-subshell. They have more complex, three-dimensional forms, often described as cloverleafs or even dumbbells. It’s like the D-subshell has a more avant-garde interior design.
It’s these five distinct orbitals, each capable of housing a pair of electrons, that grants the D-subshell its maximum capacity of ten. This is a key piece of information for scientists trying to understand the properties of elements, especially those tricky transition metals.
The fact that a D-subshell can accommodate exactly ten electrons is a testament to the elegant and precise rules that govern the atomic universe. It's like finding a perfectly fitting puzzle piece!
When we talk about electron configurations, knowing this maximum of ten for the D-subshell is crucial. It helps us predict how many electrons an atom will have in its outer shells, which, in turn, tells us a lot about its chemical personality. It's like knowing how many guests can fit in a specific room before the party starts.
So, the number ten isn't just a number; it's a fundamental aspect of atomic structure. It’s the magic number for the D-subshell, defining its potential and influencing the behavior of countless elements. It’s a hidden feature that makes the atomic world so wonderfully predictable and yet so surprisingly complex.
The Colorful World of D-Block Elements
Where do we see these D-subshells in action? They are the stars of the show in the D-block of the periodic table! These are often referred to as the transition metals, and they are responsible for so much of the colorful and interesting chemistry we observe.

Think about the vibrant colors of gemstones like emeralds and rubies. Or the shiny sheen of metals like gold and copper. Many of these properties are due to the electrons in their D-subshells. They are the artists of the periodic table!
When light interacts with atoms that have partially filled D-subshells, something magical happens. Electrons can absorb and re-emit light of specific wavelengths, giving rise to those spectacular colors we see. It's like the electrons are putting on a light show.
The fact that the D-subshell can hold up to ten electrons means that these transition metals have a lot of flexibility in how their electrons are arranged. This flexibility is key to their diverse chemical behaviors and their ability to form compounds with different oxidation states. It's what makes them so versatile.
So, the next time you admire a piece of jewelry or a strong metal structure, remember the incredible role played by the humble, yet mighty, D-subshell and its capacity for ten electrons. It’s a little detail with a big impact on the world around us.
A Glimpse into the Electron's Domain
The concept of subshells might sound a bit abstract, but it’s the underlying principle for so much of chemistry. Understanding the maximum number of electrons in a D-subshell, which is ten, is like having a key to unlock many atomic mysteries.

It’s a fundamental rule that governs how atoms interact. It’s a building block of knowledge that scientists use every day. And for us, it’s a cool fact about the tiny world of electrons that makes the universe even more amazing.
So, there you have it! The D-subshell, a fascinating electronic neighborhood, with a maximum capacity of ten electrons. It’s a number that plays a crucial role in the properties of many elements and the colorful world we inhabit.
Isn't it neat how something so small can have such a big influence? The next time you encounter a shiny metal or a vibrant pigment, give a little nod to the D-subshell and its impressive group of ten electrons. They’re working hard behind the scenes to make our world so interesting!
Perhaps this little peek into the electron's world has sparked your curiosity. There's so much more to discover about these subatomic wonders. The world of quantum chemistry is full of surprises, and the D-subshell is just one sparkling jewel in its crown.
So, keep an eye out for the D-subshell and its guest list of ten. It’s a small detail that unlocks a universe of understanding about the elements we encounter every single day. It’s a little piece of atomic magic that’s worth remembering.

The maximum number of electrons in a single D-subshell is ten. This simple fact is a gateway to understanding the diversity and complexity of the periodic table. It’s an invitation to explore further!
It's these precise numbers and structures that make science so captivating. The electron’s world, with its specific subshells and electron limits, is a testament to the order and beauty within the universe. The D-subshell and its ten electrons are a perfect example of this elegant design.
So, next time you're looking at a science textbook or a chart of the elements, remember the D-subshell. Its capacity of ten electrons is a fundamental piece of the puzzle, revealing the secrets of why elements behave the way they do. It's a small number with enormous implications.
The universe is built on these tiny, precise rules. And the D-subshell, with its limit of ten electrons, is a fantastic illustration of this. It’s a concept that, once understood, opens up a whole new way of seeing the world around us.
So, let's celebrate the D-subshell and its ability to house exactly ten electrons. It’s a small detail that contributes to the grand tapestry of chemistry and physics. And it’s a perfect starting point for anyone curious about the wonders of atoms.
