The ________ Ion Has A Noble Gas Electron Configuration.

Imagine the universe is throwing a party, and everyone wants to be in the coolest group. That's kind of like what happens with atoms! They're always trying to get their electrons in just the right spots.
And there's one specific arrangement that's super popular, like the VIP section of the atom party. It's called a noble gas electron configuration. It’s the ultimate goal for many atoms.
Think of it as having the perfect number of electrons in their outermost shell. It makes them feel complete and totally chill. They don't need to share or steal electrons anymore.
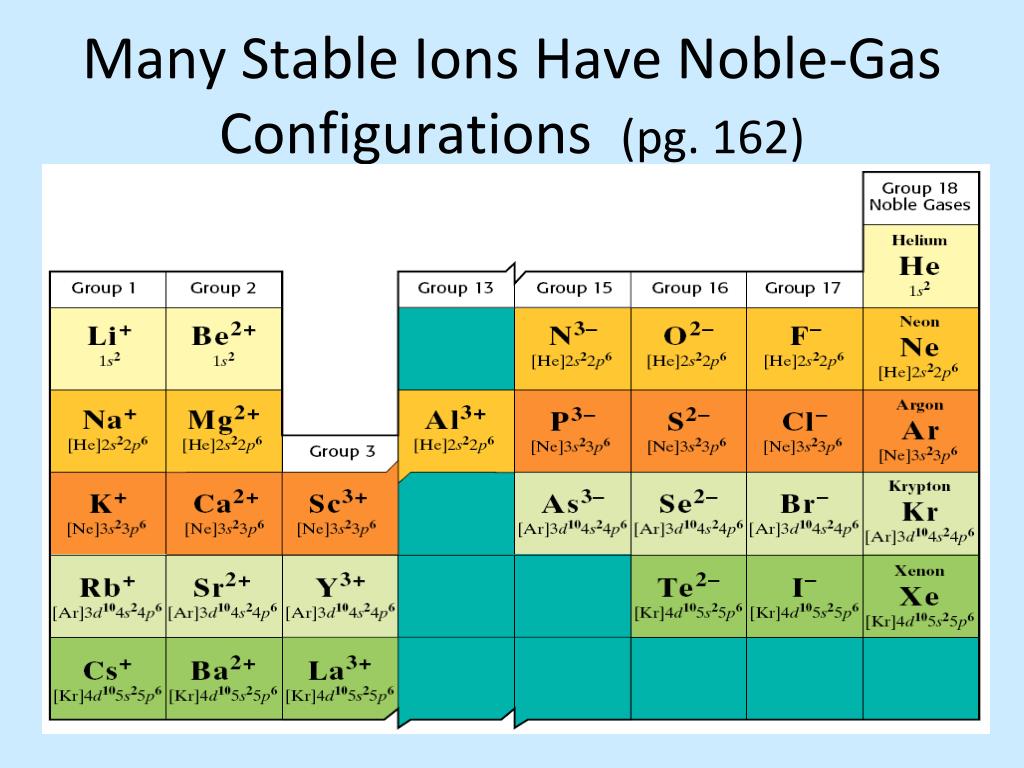
Now, guess what? There's a particular ion out there that has achieved this ultimate electron arrangement. It's the Magnesium ion! Yep, that's right.
The Mg2+ ion has managed to snag that coveted noble gas electron configuration. It's like winning the atomic lottery! This makes it a pretty special and stable character in the world of chemistry.
Why is this so cool? Well, elements usually have a bit of a personality. Some are eager to give away electrons, others are desperate to grab them. It's all about finding that balance.
But when an atom, like magnesium, becomes an ion and achieves this noble gas setup, it's like it's found its zen. It's no longer reactive in the typical way. It's pretty content.
This means the Magnesium ion doesn't easily jump into reactions to gain or lose more electrons. It's already at its happy place. This stability is what makes it so interesting.
Think about it like this: you're playing a game of musical chairs. Most people are scrambling, trying to find a seat. But the Magnesium ion is already sitting in the best chair.
It's like it's wearing a crown of electron perfection. This isn't just a minor detail; it's a big deal in the chemical world. It influences how magnesium behaves in so many situations.

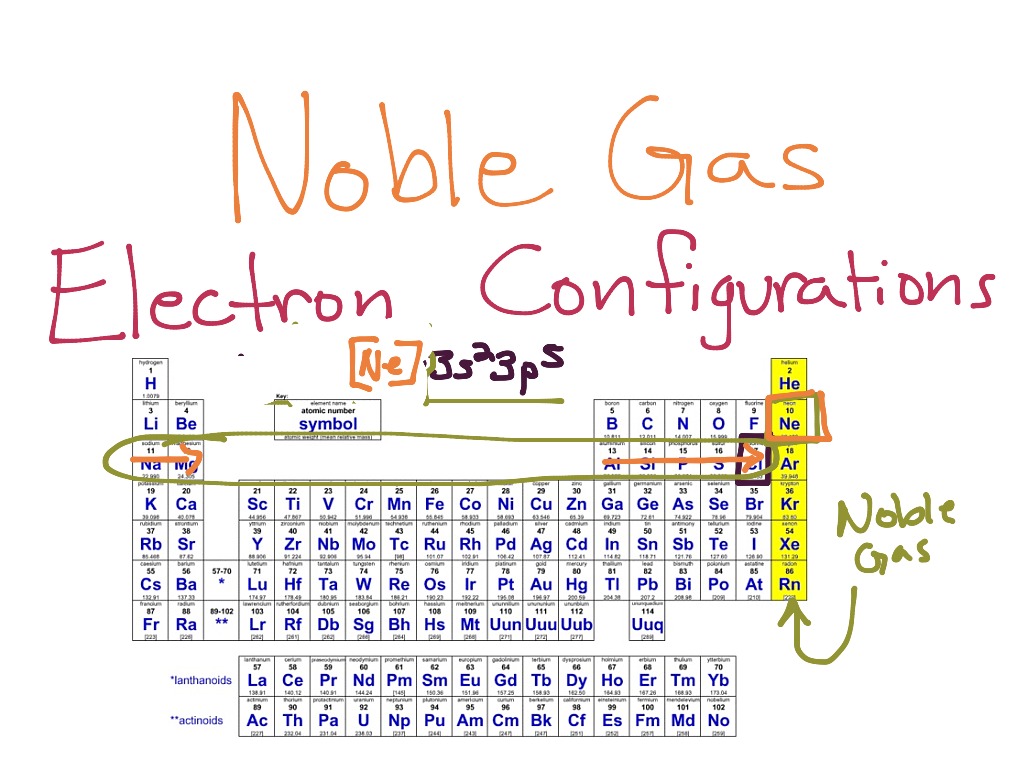
So, why does magnesium do this? Well, a neutral magnesium atom has 12 electrons. It's got a couple of electrons in its outer shell that are a little bit 'extra.'
To reach that stable noble gas configuration, it lets go of these two outer electrons. Poof! Gone! And what's left is an electron arrangement that looks exactly like Neon.
Neon is one of those super chill noble gases that just floats around, not bothering anyone. Now, the magnesium atom has transformed into the Magnesium ion, looking just like Neon. Pretty neat, huh?
This transformation is a classic example of how atoms strive for stability. It's a fundamental driving force in chemistry. And the Magnesium ion is a prime example of this principle in action.
You see magnesium in all sorts of places. It's in your bones, it helps plants grow, and it's even in fireworks! The Magnesium ion plays a crucial role in all these things.
Its stable electron configuration means it can exist in these environments without constantly reacting and changing. It's a reliable building block. It's like a sturdy Lego brick in the grand construction of life.
This stability also means that the Magnesium ion can form strong bonds with other charged particles, called anions. Think of it as finding a perfect dance partner. They stick together really well.

These ionic bonds are super important in forming compounds. Many minerals and salts are made up of ions like the Magnesium ion. They create the solid structures we see all around us.
So, the next time you hear about the Magnesium ion, remember its secret superpower: the noble gas electron configuration. It's what makes it so stable, so important, and so fascinating.
It’s like the atom equivalent of finding your perfect outfit that makes you feel amazing and confident. The Magnesium ion is dressed to impress in its electron shell.
It’s a reminder that even at the tiniest level, there are these incredible quests for balance and stability. And sometimes, that leads to some pretty awesome outcomes.
This quest for the noble gas configuration is a huge motivator for many elements. They’re always looking for that perfect electron setup. It’s a universal atomic ambition.
The Magnesium ion has just nailed it. It’s achieved that elusive state of electron bliss. It’s the rockstar of electron configurations!
It’s entertaining because it’s like watching a character in a story reach their ultimate goal. The journey of magnesium to become the Mg2+ ion is a chemical fairy tale.
It’s the triumph of stability over chaos. It's the quiet confidence of an atom that knows it's got its electron game on point.
So, while we can't see ions with our naked eyes, understanding their electron configurations opens up a whole new world of wonder. It’s like having a secret decoder ring for the universe.
And the Magnesium ion, with its noble gas electron configuration, is a shining example of this microscopic marvel. It’s a little piece of atomic perfection.
It’s a testament to the elegant simplicity that governs the universe. How a few electrons can make such a big difference to an atom's personality.
So, if you ever feel like diving a little deeper into the amazing world of atoms, remember the Magnesium ion. It's a fantastic starting point.
It’s got a story to tell about stability, transformation, and achieving that ultimate, chill electron state. It’s the VIP of the atom party, and it earned its spot.
It’s a reminder that even the smallest components of our world have their own grand dramas and triumphs. And the Magnesium ion is a star player.
It’s a little bit like a superhero achieving their perfect power-up. The Magnesium ion, powered by its noble gas electron configuration, is ready for anything.
It makes you wonder what other ions are out there with their own fascinating electron stories. The universe of atoms is full of these captivating characters.
The Magnesium ion just happens to be one of the most relatable and impressive examples. It’s a friendly face in the complex world of chemistry.
So go on, be curious! Explore the incredible science behind the tiny things that make up everything. The Magnesium ion is just the beginning of a much bigger adventure.
It’s a simple concept with profound implications. The quest for electron stability is what makes our world work, and the Magnesium ion is a perfect illustration.
It’s a little chemical magic trick that has huge consequences for how matter behaves. And it all comes down to those electrons.
The Magnesium ion has unlocked the secret to electron happiness. It's a truly noble pursuit, in more ways than one!
Think of it as the atom's version of a "happily ever after."
So next time you encounter magnesium, remember the mighty Mg2+ ion and its enviable, stable, and dare we say, noble electron configuration. It's a little piece of atomic perfection that makes our world go 'round.
