The Ground State Electron Configuration Of Ga Is

Hey there, science curious friends!
Ever wonder what makes the world tick? It's all about the tiny, invisible stuff. We're talking electrons! And today, we're diving into the super cool world of Gallium, or Ga, if you're feeling fancy. Specifically, we're gonna unravel its ground state electron configuration. Sounds technical, right? But trust me, it's way more fun than it sounds.
So, What's the Big Deal with Ga?
Gallium is this awesome metal. It's kinda weird. Like, it melts in your hand! Seriously. If you hold a tiny bit of gallium in your palm, it'll turn into a liquid. How cool is that for a party trick? Imagine the possibilities!
But beyond its melting magic, gallium plays a role in stuff like LEDs. Yep, those bright little lights? Gallium is often involved. It’s a bit of a workhorse in the tech world.
Let's Talk Electrons. They're the Real Rockstars.
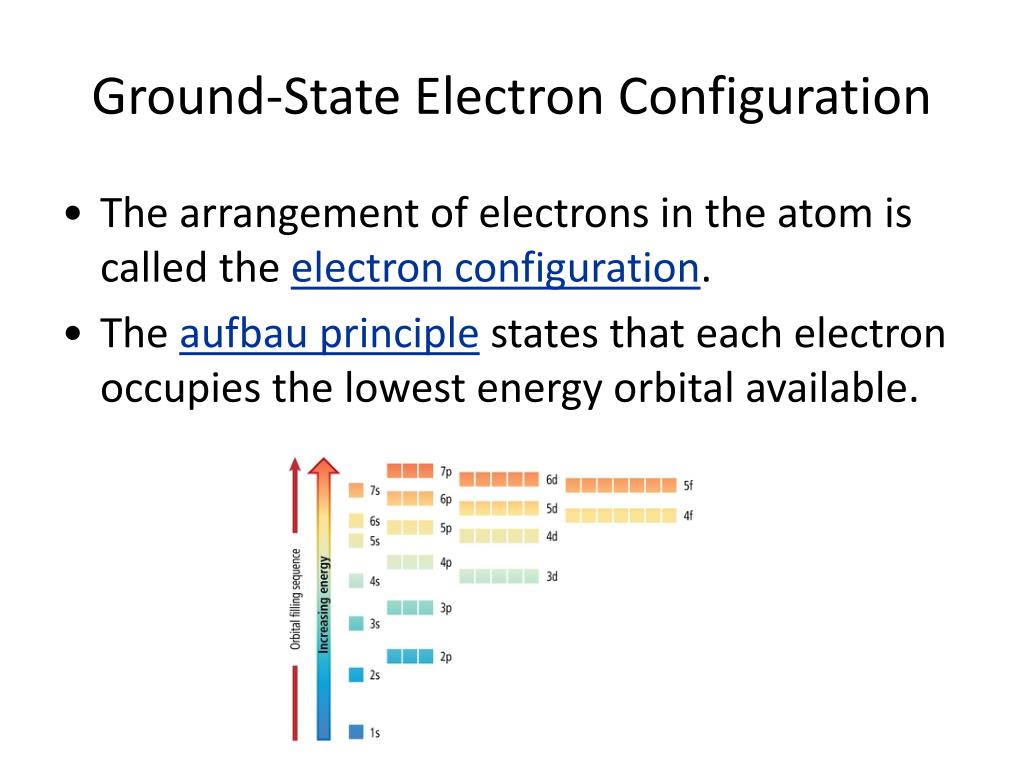
Okay, picture an atom. It’s got a nucleus in the middle, like a tiny sun. And zooming around that sun are electrons, like planets. But these planets aren't just randomly flying around. They have specific paths, like highways, called orbitals.
And these orbitals? They come in different shapes and sizes. We've got the s orbitals, which are like little spheres. Then there are the p orbitals, which are more like dumbbells. And it goes on from there!
These electrons are lazy. Or, rather, they're energy-conscious. They always want to be in the lowest possible energy state. That's what "ground state" means. It's like them chilling on the couch, not climbing any stairs. They're in their most comfortable, lowest-energy spots.
Gallium's Electron Party: The Guest List
Gallium, with its atomic number 31, has 31 electrons. That's a decent number! So, we've got 31 little electrons that need to find their perfect spot around the gallium nucleus.
We fill these orbitals up like a hotel. The lowest energy levels get filled first. Think of it like a concert hall. The front rows get filled before the nosebleeds. Makes sense, right?

The Sacred Filling Order: A Cosmic Rulebook
There's a specific order in which these orbitals get filled. It's not arbitrary. It's dictated by quantum mechanics, which is basically the super-weird, super-accurate rulebook for the tiniest things in the universe.
We use special notation to describe these orbitals. It looks a bit like a secret code: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p... and so on. The number tells you the energy level, and the letter tells you the shape of the orbital.
Let's Fill 'Em Up for Gallium!
So, let's do this. Gallium has 31 electrons. We start filling from the bottom energy levels:- The 1s orbital is like the tiniest, coziest room. It can hold up to 2 electrons. So, 1s2.
- Next up is the 2s orbital. Another little sphere, holds 2 electrons. 2s2.
- Then we have the 2p orbitals. These are a bit more exciting, like a set of three dumbbells. Each p orbital can hold 2 electrons, so three of them hold a total of 6 electrons. 2p6.
- We're on a roll! Next is the 3s orbital. Yep, another 2 electrons. 3s2.
- Then the 3p orbitals. More dumbbell action, holding another 6 electrons. 3p6.
- Now, things get a tiny bit more interesting. We hit the 4s orbital. It's a nice big sphere, and it takes 2 electrons. 4s2.
So far, how many electrons have we placed? 2 + 2 + 6 + 2 + 6 + 2 = 20 electrons. We still have 31 - 20 = 11 electrons to go!
The 3d Orbitals: A Little Complicated
After the 4s orbital is filled, the next lowest energy level is actually the 3d orbitals. This is where things get a little quirky. The 3d orbitals are like a whole bunch of more complex shapes, and there are five of them! Each can hold 2 electrons, so that's a whopping 10 electrons they can accommodate.
Now, the order of filling can seem a bit counterintuitive. Sometimes, a higher energy level's s orbital (like 4s) gets filled before a lower energy level's d orbitals (like 3d). It’s like, the 4s is a bit easier to get to for a brief moment.
For gallium, the 3d orbitals get filled next. They can hold 10 electrons. So, 3d10.
Let's tally again: 20 (from before) + 10 (from 3d) = 30 electrons. We're almost there! Just one electron left.
The Grand Finale: The 4p Orbital
The very last electron for gallium goes into the 4p orbitals. Remember those dumbbell shapes? Well, the 4p orbitals are even bigger dumbbells. They can hold up to 6 electrons, but we only have one left to place.
So, that last electron chills in one of the 4p orbitals. We write this as 4p1.
Putting it All Together: The Full Picture
So, the ground state electron configuration for Gallium (Ga) is the sum of all these filled orbitals:
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p1

Pretty neat, huh? It’s like a treasure map of where all of gallium’s electrons are hanging out when they’re at their most relaxed.
Why is This Actually Fun?
Because it's a cosmic puzzle! Each element has its own unique electron configuration, like a fingerprint. It tells us so much about how that element will behave.
That single electron in the 4p orbital? That's the one that's usually involved in chemical reactions. It's the one that gets to mingle with other atoms. It's the electron that makes gallium do its Gallium thing!
Think of it like this: The inner electrons are all cozy and protected, like they’re in their own private mansions. The outermost electrons, though? They’re out on the front porch, ready to interact. And for gallium, that's the lone 4p1 electron.
A Shortcut to Awesome: Noble Gas Notation
Writing out the full configuration can get a bit long. Scientists like shortcuts! So, we can use something called noble gas notation.
We look at the last noble gas that came before gallium in the periodic table. That's Argon (Ar), which has the configuration 1s2 2s2 2p6 3s2 3p6.

So, we can replace that whole chunk with just [Ar]. Then we just add the rest of the configuration:
[Ar] 4s2 3d10 4p1
See? Much cleaner! It’s like saying, "Okay, everything before this point is the same as Argon, so let's just skip ahead!" It’s a sciency way of saying, "The rest is history!"
The Quirky Bits Make it Interesting
The fact that the 3d orbitals fill after the 4s orbital? That's a little bit of nature's mischief. It's a reminder that the universe doesn't always follow the most obvious path. It has its own elegant, sometimes surprising, logic.
And that 4p1 electron? It’s the key to gallium’s properties. It’s what makes it a metal, what makes it able to form certain bonds. It’s the reason we can have those super bright LEDs!
So, the next time you see a gallium-based product, or just marvel at the sheer variety of elements out there, remember the humble electron configuration. It's a tiny, hidden world that dictates so much of the big world we see. And it’s just plain cool to know!
Keep exploring, keep questioning, and remember, even the most complex science can be a fun conversation starter!
