The Energy Required To Initiate An Exergonic Reaction Is Called
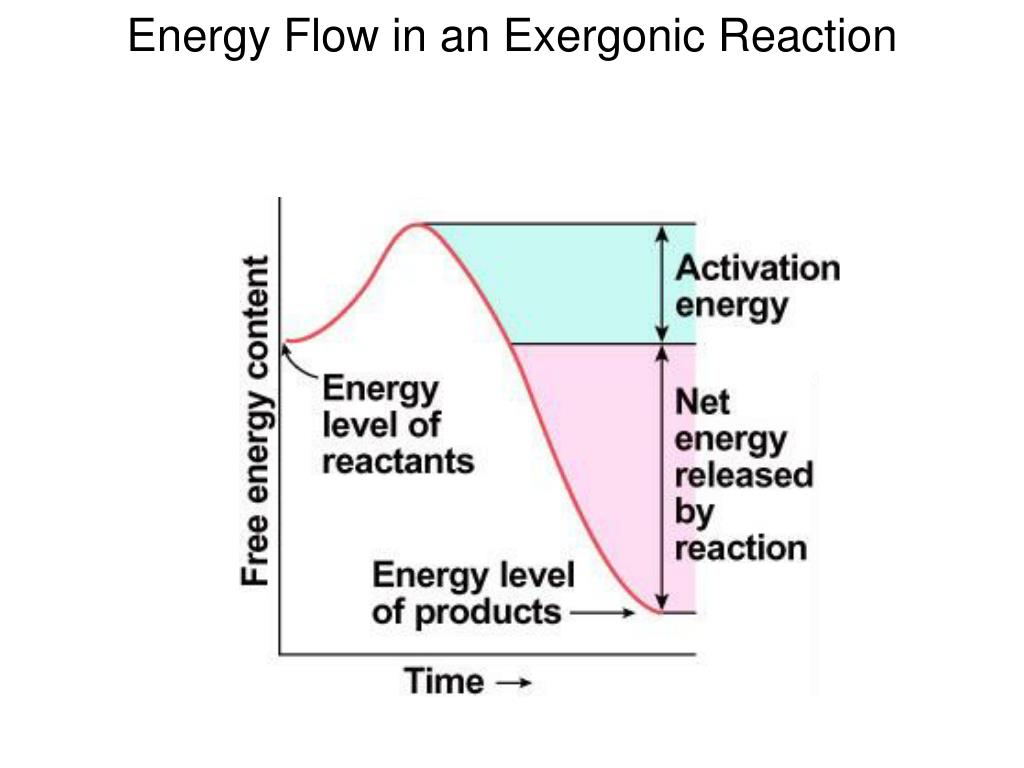
Alright, settle in, grab your imaginary croissant and a ridiculously large latte. We're about to dive into something that sounds way more intimidating than it is. Think of it as the secret handshake of chemical reactions, the tiny little nudge that gets the ball rolling. We're talking about the energy required to initiate an exergonic reaction. And no, before you start sweating, this isn't a lecture where I'll be drawing diagrams with chalk dust flying everywhere. This is more like a friendly chat over a spilled cup of coffee, with a few eyebrow-raising bits thrown in.
So, what's an "exergonic reaction"? Fancy name, right? Let's break it down. "Exo" means out, and "ergonic" is a fancy way of saying "energy-producing." So, an exergonic reaction is basically a chemical reaction that gives off energy. Think of it like a little chemical firecracker that goes off and releases a burst of heat or light. It’s the universe saying, "Here, have some free energy! Go wild!"
But here's the funny part, the real kicker: even these energy-releasing superstars don't just spontaneously combust. Nope. They need a little… encouragement. They need a little push. They need what scientists, in their infinite wisdom and love for long words, call the activation energy.
Imagine you're trying to get a boulder to roll downhill. You know, once it starts, it'll just keep going, gathering speed and making a delightful mess. That's an exergonic reaction for you. But that boulder isn't going to spontaneously sprout little legs and scoot itself over the edge, is it? No. You, my friend, have to give it a shove. That shove? That's the activation energy.
It's like when you're trying to get out of bed on a Monday morning. Your bed is super comfy, right? Super low energy, all cozy. But the world outside that duvet? Full of potential energy, tasks, maybe even that questionable office coffee. Getting out of the bed, that first stretch, that first groggy step – that's your activation energy. Once you're up and walking, your body starts generating its own energy, fueled by breakfast and sheer desperation. See? You're an exergonic machine!
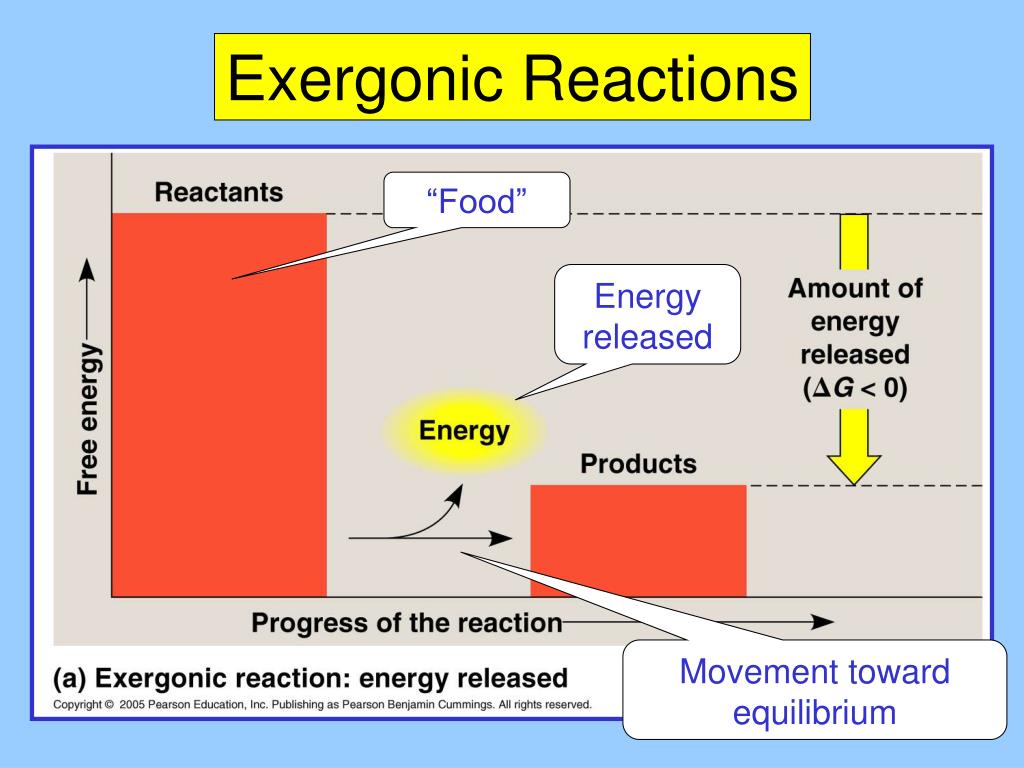
So, the activation energy is the initial energy investment you need to make to kickstart a reaction that will eventually give you more energy back. It's like paying a tiny entrance fee to a party that's going to be legendary. You put in a little bit to get a whole lot of fun (and energy) out.
Why does this even happen? Well, think about the molecules involved. They're like shy dancers at a party. They're surrounded by other molecules, but they're not quite ready to mingle, to bond, to break apart and reform into something new. They're stable, chilling in their own little molecular bubbles. To get them to actually do something, to react, you need to give them a jolt. You need to give them enough energy to overcome their hesitations, to break some existing bonds, and to reach a state where they can then form new, more stable bonds, releasing that glorious energy.
This "state" they have to reach is called the transition state. It's like the awkward moment at the party when someone tries to start a dance-off. Everyone’s a bit hesitant, nobody’s sure who’s going to make the first move. The transition state is that moment of intense awkwardness before the real fun begins. It's a fleeting, high-energy, unstable state. And to get there, you need that activation energy.
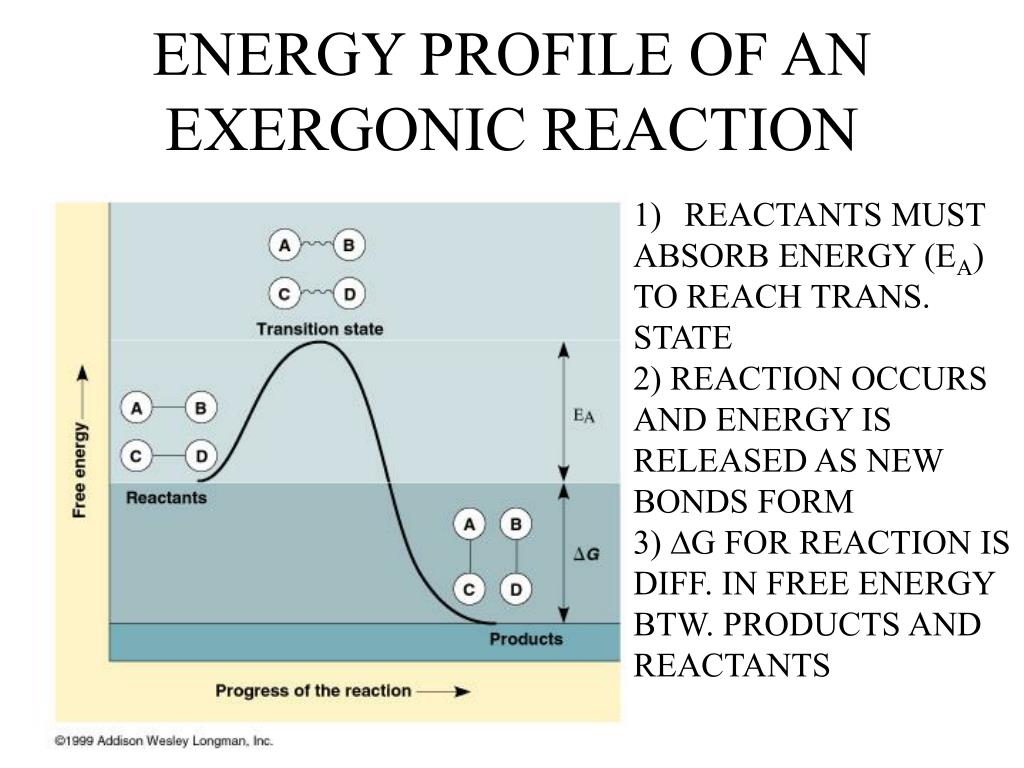
Think of a perfectly ripe avocado. It's got all this deliciousness locked up inside, ready to be mashed into guacamole. But it's not going to magically turn into guacamole on its own. You need to pick it up, cut it, pit it, and mash it. The act of cutting and pitting? That's your activation energy. Once you've done that, you can then mash it and enjoy the delicious, energy-releasing process of making guacamole. (Okay, the guacamole itself doesn't release energy in the chemical sense, but my point about the effort stands! It’s a culinary analogy, not a chemistry thesis.)
Now, here’s a surprising fact: sometimes, even reactions that release a TON of energy can have a surprisingly high activation energy. Like, imagine a pile of dry tinder. You know that if you strike a match, it's going to go up in flames and produce a whole lot of heat. But you still need that tiny spark, that initial flame from the match. Without it, the tinder just sits there, looking dry and… well, not on fire.

This is also why we don't spontaneously combust. Our bodies are a constant hive of exergonic reactions – like our cells converting food into usable energy. But these reactions are happening in a very controlled way. We have special molecules called enzymes that act like tiny molecular matchmakers. They lower the activation energy needed for these reactions to occur, making them happen quickly and efficiently at body temperature. Without enzymes, our metabolic reactions would need so much energy to get started that we'd basically be… frozen in time, or at least very, very sluggish.
So, the energy required to initiate an exergonic reaction, this essential little shove, this tiny spark, this Monday morning get-out-of-bed effort, is called the activation energy. It's the price of admission to the energy party. It’s the hero’s journey for molecules. It’s the reason why things don’t just… do stuff… without a little bit of persuasion. And in the grand, chaotic theatre of chemistry, it’s a surprisingly small, yet utterly crucial, player.
Next time you see something happen, a chemical reaction, a bodily process, even a toddler starting a tantrum (which, let's be honest, is often an exergonic event), remember that little boost of energy it needed to get going. It's the unsung hero of the chemical world, and frankly, it deserves a tiny applause. Or at least a really good cup of coffee.
