The Cyclohexane Ring Is Essentially Free Of Ring Strain Because

Okay, let's talk about something a little… cyclic. We're diving into the world of chemistry, but don't let that scare you! We're not building rockets here. Think of it more like a slightly nerdy, but totally delightful, chat about a common molecule.
This molecule is called cyclohexane. You might have seen it in chemistry textbooks. It’s shaped like a ring, which sounds fancy, right? But honestly, it's pretty chill.
Now, the big question, the one that might even spark a little debate among the lab coat crowd, is this: why is this cyclohexane ring basically… free of ring strain? It sounds complicated, but stick with me.
Imagine you have a bunch of friends holding hands in a circle. If they have to squeeze too close, or stretch too far, it’s uncomfortable. That’s kind of like ring strain in molecules. It’s when the atoms in a ring are forced into awkward angles.
But cyclohexane? It’s like that group of friends who just… fit perfectly. They’re holding hands, everyone’s comfortable, and nobody’s complaining. It's a happy circle.
The reason is quite brilliant, really. It all comes down to the way the carbon atoms are arranged and how their bonds behave. They’re not being bossed around by some rigid geometry.
Think of it this way: if you try to make a square out of popsicle sticks, it works. But if you try to make a triangle with those same sticks, you’re going to have to do some bending. The angles are just naturally different.
With cyclohexane, the molecule is smart enough to avoid these awkward angles. It’s like it found the perfect dance move to keep everyone happy and relaxed. No forced smiles here!
The cyclohexane ring isn't flat, you see. That’s a crucial detail! If it were a perfectly flat hexagon, like a drawing on a piece of paper, it would be a different story.
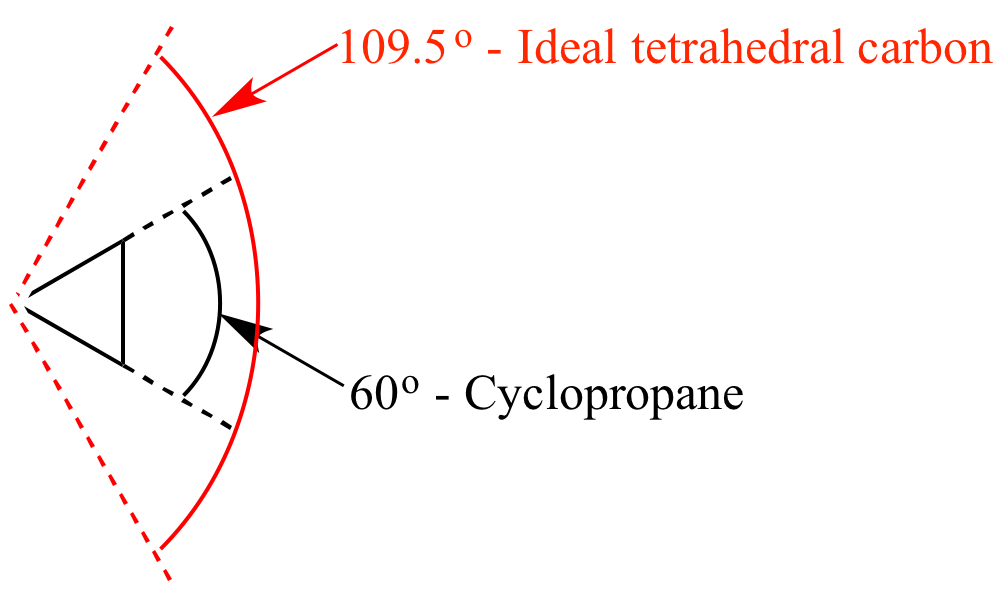
A flat hexagon would mean the bond angles are all stuck at 120 degrees. For carbon atoms bonded like this, that’s just not their happy place. It’s like trying to wear shoes that are two sizes too small – just not going to happen without some pain.
So, how does cyclohexane get around this? It twists and turns. It adopts a shape that’s much more comfortable for its atoms. It’s not trying to be something it’s not.
The most stable shape, the one that makes everyone in the ring sigh with relief, is called the chair conformation. Say it with me: "chair conformation." It sounds cozy, doesn’t it?
In this chair conformation, the carbon atoms aren't all lined up in a predictable, strained pattern. Instead, they sort of… ripple. Some are up, some are down, mimicking the relaxed posture of, well, a comfy chair.
This twisting and turning allows the bond angles to be much closer to the ideal 109.5 degrees. That’s the sweet spot for carbon-carbon single bonds. It’s like finding that perfect temperature – not too hot, not too cold.
And it’s not just the bond angles that are happier. The hydrogens attached to the carbons are also having a grand old time. They’re not bumping into each other unnecessarily.
You know when you're in a crowded elevator, and everyone's awkwardly bumping elbows? That's like strain in a molecule. But in cyclohexane's chair, the hydrogens are spaced out nicely.

There are two types of hydrogens in the chair conformation: axial and equatorial. Don't let the big words intimidate you. Think of them as up-and-down hydrogens (axial) and out-to-the-side hydrogens (equatorial).
In the chair, these hydrogens are arranged so they don’t get in each other’s way too much. There are some minor bumps, sure, but they're so small they’re practically whispers. No shouting matches here.
This freedom from major strain is why cyclohexane is such a common and stable molecule. It’s the reliable friend in the molecular world. It just… works.
So, while other rings might be all tense and uncomfortable, cyclohexane is over here, lounging in its chair, completely at ease. It’s like the molecule that’s figured out the secret to a stress-free existence.
It’s almost an unpopular opinion, isn't it? To say that a ring is basically strain-free. Many chemists would nod along, but for the rest of us, it's a fascinating little tidbit.
It's the elegance of nature, really. How atoms can arrange themselves in just the right way to achieve maximum comfort and stability. It’s a lesson we could all learn from.

So next time you think of cyclohexane, don’t picture some rigid, uncomfortable shape. Picture a comfy chair, a happy circle of friends, and a molecule that’s just vibing.
It’s the subtle genius of chemistry. The way things just fit. And for cyclohexane, that fit is so good, the strain is practically a myth. A very small, easily ignorable myth.
It's like that one friend who always knows how to relax. They’ve got the perfect posture, the right amount of space, and they just exude calmness. That's our cyclohexane.
And the best part? This is all happening at a microscopic level, with atoms and bonds. It’s a whole tiny universe of comfort and good design.
So, there you have it. The cyclohexane ring, living its best, strain-free life. It’s a testament to how molecules can find their perfect form, without all the fuss.
It's not trying to bend itself into impossible shapes. It's not forcing its atoms into awkward hugs. It's just naturally comfortable.
And that, my friends, is why cyclohexane is basically free of ring strain. Because it’s a smart cookie, and it knows how to relax.
It’s a simple concept, really, when you break it down. No need for complicated equations to appreciate a well-designed molecule. Just a little bit of imagination.
So, the next time you encounter cyclohexane, give it a nod of appreciation. It's a molecule that has truly mastered the art of being comfortable.
It's the unbothered king of cyclic hydrocarbons. It's not sweating the small stuff, or the big stuff, for that matter.
And that’s precisely why, in the grand scheme of molecular rings, cyclohexane is practically floating on a cloud of relief. No strain, just chill.
It’s the elegance of nature, really. How atoms can arrange themselves in just the right way to achieve maximum comfort and stability.
It’s a molecule that has it all figured out. The angles are right, the hydrogens are happy, and the ring is as stable as a well-built house.
So, let’s raise a metaphorical toast to cyclohexane, the champion of comfortable rings. It's a molecule that proves that sometimes, the best way to avoid problems is simply to be perfectly designed.
And that, in a nutshell (or perhaps a comfortable chair conformation), is the story of why cyclohexane is so wonderfully, blissfully, and almost unbelievably, strain-free.
