Table Salt Forms From Sodium And Chloride Via Hydrogen Bonding.

Hey there, ever wondered about that humble little shaker of table salt sitting on your kitchen counter? It’s a kitchen superhero, right? We sprinkle it on everything to make food taste amazing. But have you ever stopped to think about where it actually comes from? It’s not just magically appearing in those neat little crystals!
Well, get ready for a fun little science adventure because today we’re talking about how table salt is born. And trust me, it’s way cooler than you might think. It involves a super exciting team-up between two rather chatty characters: Sodium and Chloride. They’re like the rock stars of the chemical world!
Now, Sodium sounds a bit like it could be a superhero name, doesn't it? But in reality, plain old Sodium (you know, the pure stuff) is actually a bit of a wild and crazy element. It's so reactive, it's like a little kid with way too much sugar! It loves to grab things and get involved in all sorts of reactions.
On the other hand, we have Chloride. Think of Chloride as the slightly more reserved, but equally important, half of this dynamic duo. It's not quite as energetic as Sodium, but it's got its own special vibe. And when these two meet, sparks (quite literally, sometimes!) fly.
So, how do these two come together to form the delicious salt we know and love? It's a process that’s not only fascinating but also uses a clever trick of nature called hydrogen bonding. Yep, you heard that right! Hydrogen bonding sounds like something out of a chemistry textbook, but it’s actually a key player in this whole salt-making drama.
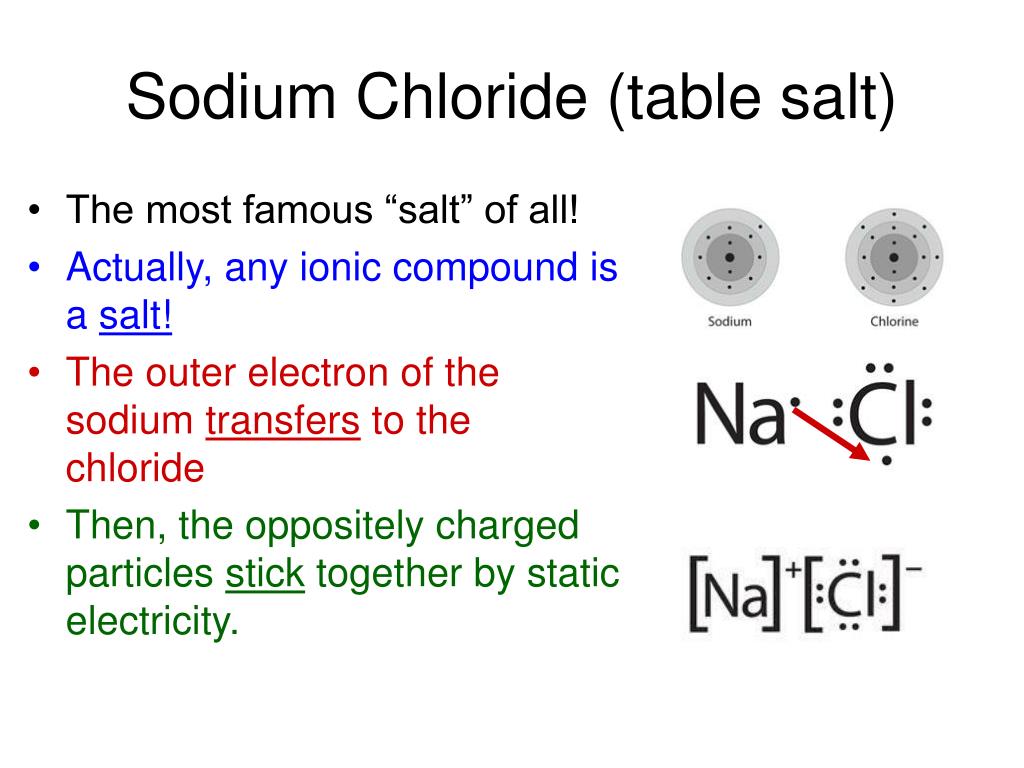
Imagine Sodium and Chloride as two friends who are looking for a perfect partner. Sodium is like a positively charged magnet, always looking to give away something to feel complete. Chloride, on the other hand, is like a negatively charged magnet, desperately wanting to receive something to feel whole.
When these two meet, it’s a match made in elemental heaven! Sodium generously gives away one of its tiny little particles, called an electron. This makes Sodium happy and gives it a positive charge. And guess who’s thrilled to get that electron? You guessed it – Chloride!
By accepting the electron, Chloride becomes negatively charged. So, now we have Sodium with a plus sign and Chloride with a minus sign. They're like two puzzle pieces that perfectly fit together, but with a little electric twist!
This attraction between the positively charged Sodium and the negatively charged Chloride is super strong. It’s an ionic bond, a powerful handshake that holds them tightly together. But here’s where hydrogen bonding adds its own special magic. While the main attraction is between Sodium and Chloride, the way these ions arrange themselves and interact with water molecules (which are always hanging around!) involves these subtle, yet significant, hydrogen bonds.
Think of hydrogen bonds as little whispers of attraction between water molecules and the salt ions. Water molecules are a bit like tiny magnets themselves, with positive and negative ends. They get attracted to the charged Sodium and Chloride ions, helping to pull them apart from their original forms and keep them dissolved and happy in water.
When Sodium and Chloride come together in the right conditions, they start to line up. They form a repeating pattern, like building blocks stacking on top of each other. This orderly arrangement is what creates those beautiful, geometric crystals of table salt we see.
Each Sodium ion is surrounded by Chloride ions, and each Chloride ion is surrounded by Sodium ions. It’s a perfectly balanced dance of positive and negative charges. This is what gives salt its characteristic crystalline structure.
The way these ions interact, especially in the presence of water during its formation or dissolution, is where hydrogen bonding plays a crucial supporting role. It’s not the main handshake, but it’s like the friendly nod between neighbors, helping everything to settle into place and behave nicely.
So, this isn't just a simple chemical reaction; it's a beautiful collaboration. Sodium, in its energetic form, finds its perfect partner in Chloride. And their strong attraction, influenced by the subtle power of hydrogen bonding with surrounding water, leads to the creation of something so fundamental to our lives.
It’s kind of like when two best friends meet and realize they just click. They balance each other out, and their combined energy creates something new and wonderful. In this case, that something new is sodium chloride, or table salt!

The formation of salt is a reminder that even the simplest things in our kitchens have an incredible story behind them. It’s a story of elements finding each other, forming bonds, and arranging themselves in a perfect structure.
And the role of hydrogen bonding? It’s the unsung hero, quietly helping to stabilize and organize these interactions, especially when water is involved. It’s like the friendly background music that makes the whole concert sound amazing.
Next time you reach for that salt shaker, take a moment to appreciate the journey these elements have taken. From volatile Sodium and receptive Chloride, to the powerful ionic attraction and the subtle influence of hydrogen bonding, it's a true marvel of chemistry.
It’s a little bit of everyday magic, happening right in front of us. The science behind table salt is a fantastic example of how elements interact to create compounds that are essential to life and taste.
So, isn’t that just neat? You’re not just adding flavor; you’re adding a sprinkle of elemental history to your meals. It makes you wonder what other everyday items have such cool origin stories, doesn't it?
The way Sodium and Chloride bond is fundamental to why salt behaves the way it does. It dissolves in water, it forms those sharp edges, and it enhances flavor. All thanks to this amazing partnership.
And that, my friends, is the entertaining tale of how table salt is formed. A little bit of sizzle, a lot of attraction, and a touch of subtle bonding. It's science, but it's also pretty darn cool!

So, the next time you're seasoning your fries or your soup, remember the journey of Sodium and Chloride. They’ve come a long way to make your food taste so good!
It's a reminder that the world around us, even in the most common objects, is full of amazing stories waiting to be discovered.
Isn’t it exciting to think that the salt on your table is the result of such a precise and elegant chemical dance?
The interplay between Sodium, Chloride, and the subtle nudges of hydrogen bonding is a perfect example of how elements work together.
This ionic bond, strengthened and organized by factors like hydrogen bonding with water, is the secret behind salt’s structure.
It’s a simple compound, yes, but its formation is a complex and beautiful process.
+is+an+example+of+ionic+bonding%2C+that+is%2C+electron+transfer+among+atoms+or+redox+reaction..jpg)
So, go ahead, sprinkle away! You’re not just adding flavor; you’re adding a touch of elemental wonder to your life.
The story of table salt is a testament to the power of attraction and the intricate ways matter comes together.
It’s a delightful chemistry lesson disguised as a kitchen staple.
And the way those ions arrange themselves, influenced by hydrogen bonds, is a sight to behold in the microscopic world.
This bonding is what makes salt so stable and so useful!
So, remember the next time you’re reaching for the salt, you’re tapping into a fascinating chemical story!
It's a true testament to the power of elemental interactions!
