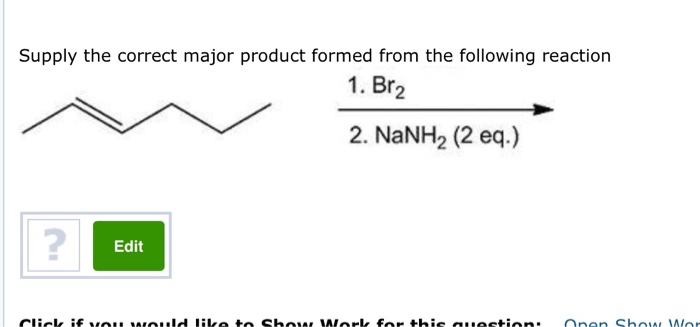
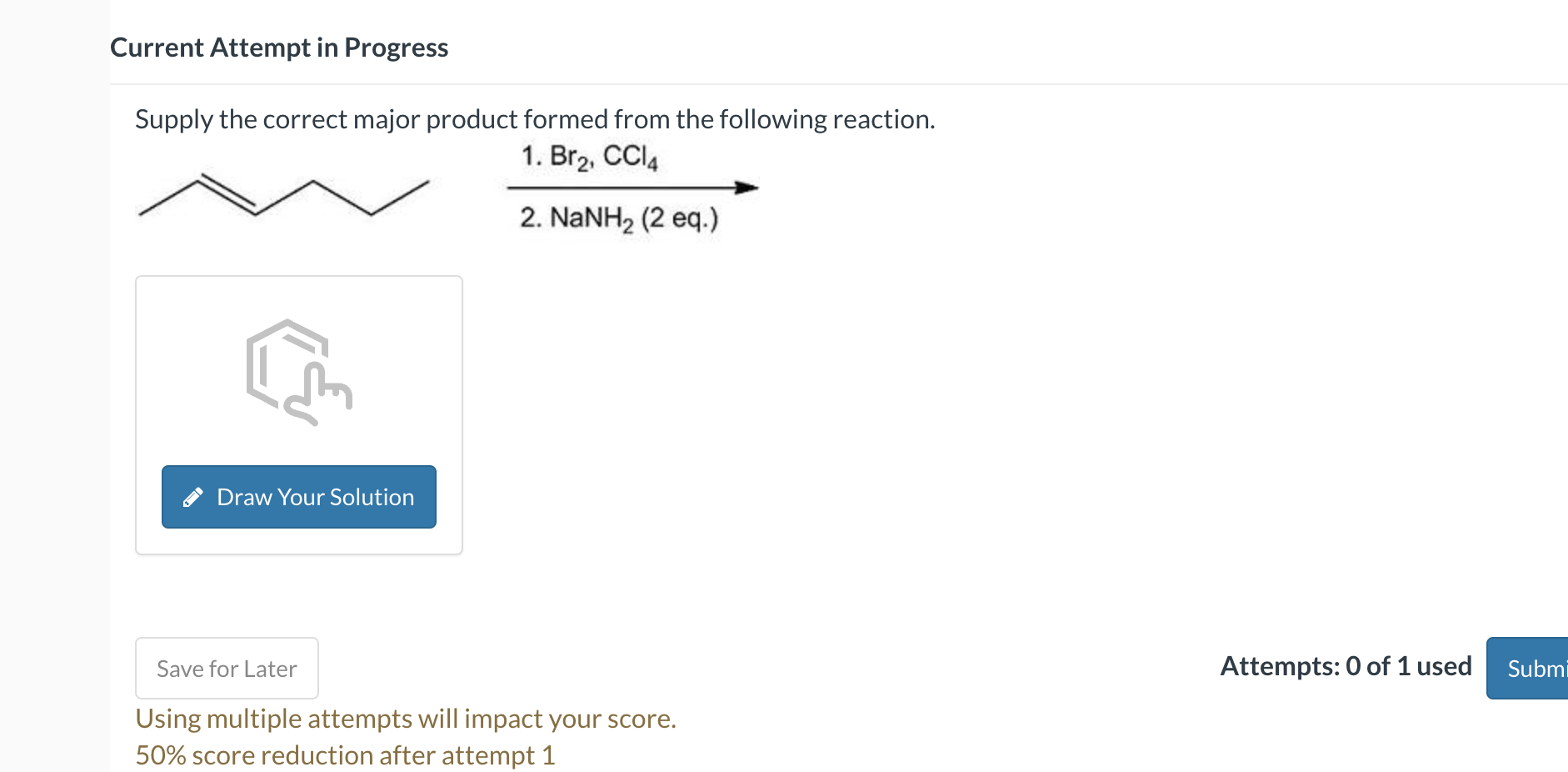
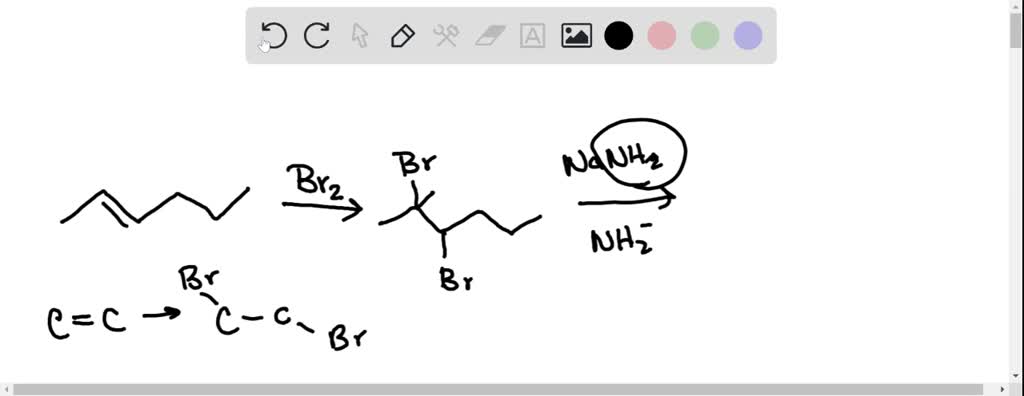
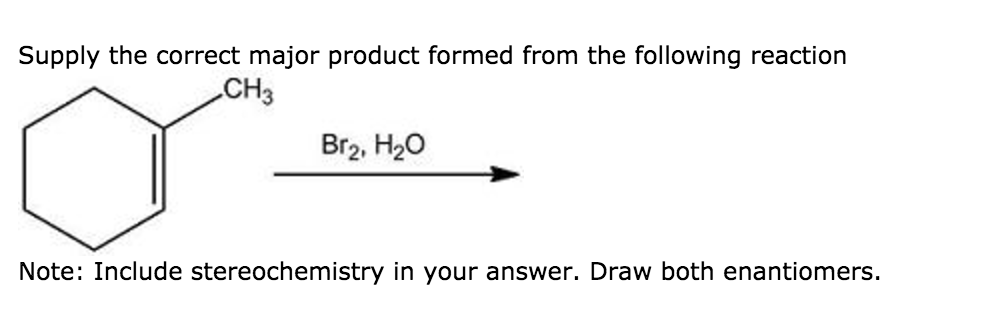
Supply The Correct Major Product Formed From The Following Reaction
Alright, so imagine this: you’ve got some chemicals chilling, and then BAM! A reaction happens. It’s like a tiny, super-fast party in a test tube. Today, we're gonna peek at one of these chemical shindigs and figure out what cool new thing pops out. No need to grab your lab coat, just your curiosity. We're diving into the wacky world of organic chemistry, and trust me, it’s more fun than it sounds. Think of it like a puzzle, but with atoms and molecules instead of cardboard pieces.
So, what’s the big mystery? We're talking about a reaction that’s all about adding stuff. Specifically, we're adding a halogen. You know, like chlorine or bromine? They’re pretty reactive little guys. And we're adding it to something called an alkene. Alkenes are those molecules with a double bond. Think of that double bond as a handshake that’s really easy to break. It’s like they’re just begging for something to join in on the fun.
The reaction we’re looking at is called electrophilic addition. Sounds fancy, right? But it’s actually pretty straightforward. The halogen, let’s call it "Hal," is feeling a bit positive. It’s an electrophile, which basically means it’s looking for some electrons to hang out with. And where are the electrons hanging out? Yup, in that juicy double bond of the alkene. It’s like a magnet, or, dare I say, a chemical mosh pit where Hal jumps in and grabs some electrons.
So, Hal, being the eager beaver it is, attacks that double bond. It’s like it’s saying, "Hey there, double bond! Mind if I join the party?" The double bond, being all flexible and ready to react, breaks open. One of the atoms from Hal latches onto one of the carbons in the broken double bond. And guess what? This leaves the other atom from Hal with a negative charge. We call that a halide ion. It’s like Hal brought a friend, and the friend is feeling a little left out, but in a good way, because it’s ready to join the next step!
Now, this isn't just a one-part show. After Hal gets cozy with the alkene, something else happens. That negatively charged halide ion we just made? It's now looking for a positive spot. And where do you think it finds one? You guessed it! It goes right to the other carbon atom that used to be part of the double bond. It’s like the perfect match, a chemical soulmate. The halide ion connects to that carbon, and voilà! We’ve got ourselves a new molecule.
What kind of molecule are we talking about here? This reaction is all about halogenation. We're adding a halogen to an alkene. The result? A dihaloalkane. That means we now have two halogen atoms attached to the carbon chain. Pretty neat, huh? It’s like taking a simple, double-bonded molecule and giving it a couple of extra, shiny halogen decorations. Imagine an alkene as a plain t-shirt, and the dihaloalkane is that same t-shirt with two cool pins attached.
But here's where things get a little more interesting, and a lot more fun. What if the alkene isn't symmetrical? What if it’s got different numbers of hydrogens on each side of the double bond? This is where a cool rule comes into play called Markovnikov's Rule. It's like the chemist's version of "finders keepers." In this case, the hydrogen atom from the reagent (if we were adding something like HBr, for example) would go to the carbon that already has more hydrogens. It’s like the hydrogen is saying, "I’ll go where I’m most welcome, where there’s already a crowd!"
Now, if we're just adding a halogen molecule itself (like Cl₂ or Br₂), we don't have that hydrogen to worry about following Markovnikov's Rule. The halogens are pretty much equally happy to attach themselves to either carbon of the double bond. The real fun starts when we have a more complex alkene, or when we're adding something like HBr or HCl. But for this specific scenario, where we're just thinking about the major product from adding a halogen (like Cl₂ or Br₂), it's pretty straightforward. The two halogen atoms will end up on the two carbons that were originally part of the double bond.
Think about ethene, the simplest alkene. It’s just two carbons with a double bond and four hydrogens. If you add chlorine (Cl₂) to ethene, you get 1,2-dichloroethane. Two chlorines, one on each carbon that was in the double bond. Simple as that. Now, imagine a slightly more complicated alkene. Let’s say we have propene. That’s three carbons, with the double bond between the first two. If we add chlorine to propene, the two chlorine atoms will attach to the first two carbons. One chlorine on carbon 1, and another on carbon 2. That’s 1,2-dichloropropane. Still pretty predictable.
The reason we call it the major product is because sometimes, in the chaotic world of chemistry, tiny amounts of other things can happen. But the overwhelming, you-can-bet-your-bottom-dollar product is the one where both halogens have joined the party on the carbons that used to be double-bonded. It’s like a handshake that’s been completed with two new hands joining in.
Why is this cool? Well, these dihaloalkanes are super useful building blocks. They’re like the LEGOs of organic chemistry. You can take them and do all sorts of other reactions to make even more complex and interesting molecules. Think pharmaceuticals, plastics, all sorts of everyday things start with reactions like these. It’s the beginning of the chain reaction of creation, if you will!
And it's just plain fun to imagine. Atoms dancing, bonds breaking and forming, all happening at lightning speed. It’s a microscopic ballet, a chemical fireworks show. The elegance of it all, how these simple rules lead to predictable outcomes, is truly something to marvel at. It’s a little piece of order in what can sometimes seem like a chaotic universe.
So, the next time you hear about a chemical reaction, don't picture a scary, bubbling beaker. Picture a party. A very organized, very exciting party where molecules are the guests of honor, and the products are the amazing souvenirs. And in this case, the souvenir is a dihaloalkane, a molecule that's just a little bit more decorated, a little bit more ready for its next adventure.
It’s a fundamental reaction, sure, but understanding it unlocks a whole universe of possibilities. It’s like learning your ABCs before you can write a novel. These basic additions are the foundation for so much more. And that, my friends, is why even a simple halogen addition to an alkene is a topic worth geeking out about. It’s the start of something big, a tiny step that leads to giant leaps in science and technology. Pretty wild when you think about it, right?
