Sodium Carbonate + Sulfuric Acid Complete Ionic Equation
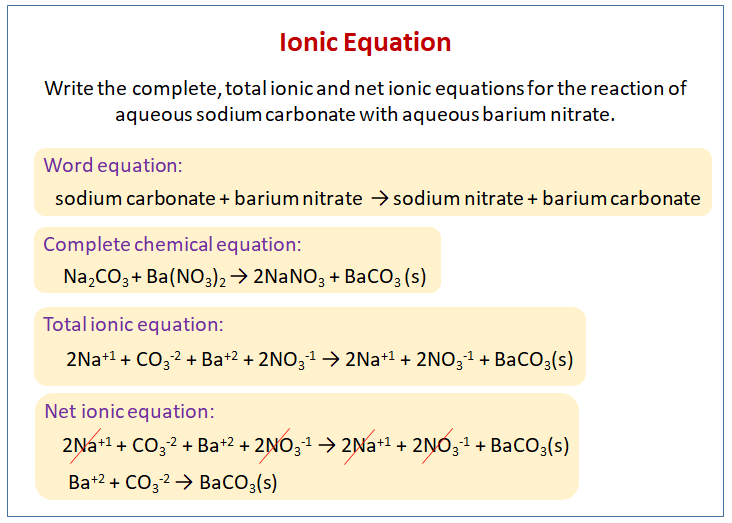
Imagine a bustling party in a beaker. We've got some very popular guests: Sodium Carbonate, also known as washing soda, who’s always bringing the bubbly fun, and Sulfuric Acid, a strong character who means business. They’re about to have quite the eventful get-together!
Now, normally, when these two meet, things get a little… fizzy. Think of it as an excited handshake that releases a lot of energy. It’s a chemical reaction, and in the world of chemistry, reactions are like little stories unfolding before our eyes.
Sodium Carbonate is a compound that loves to break apart when it’s in water. It’s like a group of friends who are always holding hands, but in water, they let go and become individual characters. There are two happy sodium ions, Na⁺, and one carbonate ion, CO₃²⁻, each looking for something new to do.
Then there’s Sulfuric Acid. This one is a bit of a drama queen. When you put it in water, it immediately splits into two enthusiastic hydrogen ions, H⁺, and a sulfate ion, SO₄²⁻. It’s quite eager to mingle!
When the Sodium Carbonate partygoers and the Sulfuric Acid partygoers all find themselves in the same space, a lot of introductions happen very quickly. It’s a bit like a crowded dance floor where everyone is bumping into each other and forming new partnerships.
The happy Na⁺ ions are pretty chill. They’re like the friendly bystanders at a party, not really looking to get too involved in the main drama. They just float around, happy to be there. They end up bonding with the SO₄²⁻ ion, forming a new compound.
This new compound is called Sodium Sulfate. It’s like a new couple forming at the party, a stable and pleasant pairing. It’s also very good at dissolving in water, so it continues to hang out in its individual ion form.
But the real stars of this particular reaction are the H⁺ ions from the sulfuric acid and the CO₃²⁻ ion from the sodium carbonate. They have a much more energetic and exciting interaction.
When an H⁺ ion meets a CO₃²⁻ ion, it's like a spark igniting. They team up to form something called carbonic acid, H₂CO₃. This is a rather unstable character at the party, not one to stick around for long.
Think of carbonic acid as that friend who tells a really funny joke and then immediately needs to leave the room because they’re laughing too hard. It’s too much excitement for one molecule!
So, this newly formed carbonic acid, H₂CO₃, decides it’s time for another little party trick. It very quickly breaks down into two even simpler things: water, H₂O, and carbon dioxide gas, CO₂.
And this is where the real fun begins! The CO₂ is the reason for all the fizzing and bubbling we see. It’s like the party guests are letting out little sighs of excitement, and these sighs escape as tiny bubbles of gas.
The carbon dioxide gas, CO₂, is very eager to get out of the liquid. It’s the guest who’s been waiting for their turn to shine, and now they’re making their grand exit, carrying all that bubbly energy with them. This is what makes the reaction so visually dramatic!
The water, H₂O, is the calm and collected one. It’s the foundation of the party, always there, remaining in its liquid form. It’s like the host who makes sure everyone has enough to drink and is comfortable.
So, when we look at the complete ionic equation, it’s like writing down the guest list and all the little interactions happening at this chemical party. It shows every single ion that’s present and how they are interacting with each other.
We start with our guests: two Na⁺ ions, one CO₃²⁻ ion, two H⁺ ions, and one SO₄²⁻ ion. They are all floating around, ready to mingle.
Then, we see the new friendships forming. The two Na⁺ ions pair up with the one SO₄²⁻ ion to create Sodium Sulfate, which then breaks apart again into two Na⁺ ions and one SO₄²⁻ ion. They are the quiet observers, forming stable, dissolved pairs.
The more exciting part involves the two H⁺ ions and the one CO₃²⁻ ion. They temporarily form carbonic acid, H₂CO₃. But as we mentioned, this is a fleeting moment of drama.

This carbonic acid, H₂CO₃, immediately decomposes into two H₂O molecules and one CO₂ molecule. This is where the bubbles come from, the visible sign of the reaction!
So, the complete ionic equation shows us everything that’s happening, from the initial guests to the final products. It’s a snapshot of the entire chemical dance.
If we were to write it out, it would look something like this:
2Na⁺(aq) + CO₃²⁻(aq) + 2H⁺(aq) + SO₄²⁻(aq) → 2Na⁺(aq) + SO₄²⁻(aq) + H₂O(l) + CO₂(g)
Look closely! Notice how some ions are on both sides of the equation. These are the spectator ions – the Na⁺ and SO₄²⁻. They are like the guests who came to the party, mingled a bit, but ultimately didn’t change their fundamental nature. They are just there, observing the main events.
They are present in the solution both before and after the reaction, almost like they’re just chilling on the sidelines, completely unaffected by the main drama. They are the steady presence in the bustling chemical soirée.
The real transformation happens with the hydrogen ions (H⁺) and the carbonate ions (CO₃²⁻). They are the ones who go through the most significant change, forming new substances like water and carbon dioxide.
It’s their interaction that drives the observable changes, like the delightful effervescence we associate with this chemical reaction. They are the life of the party, creating the excitement!
The final products are water (H₂O), which is the unsung hero of many reactions, and carbon dioxide (CO₂), the star of the show with its dramatic bubbly exit. These are the new entities that emerge from the initial mingling.
So, the next time you see something fizzing and bubbling, remember the party that’s happening inside! It’s a fascinating dance of ions, where some just watch, and others create a grand, bubbly spectacle.
This reaction, while seemingly simple, is a beautiful illustration of how different substances interact. It’s a reminder that even in the world of chemistry, there are stories of transformation and excitement, all happening on a microscopic level.
It’s the everyday magic that occurs when you mix a little bit of cleaning power with a strong acidic character. A little bit of chemistry can certainly make things interesting!
