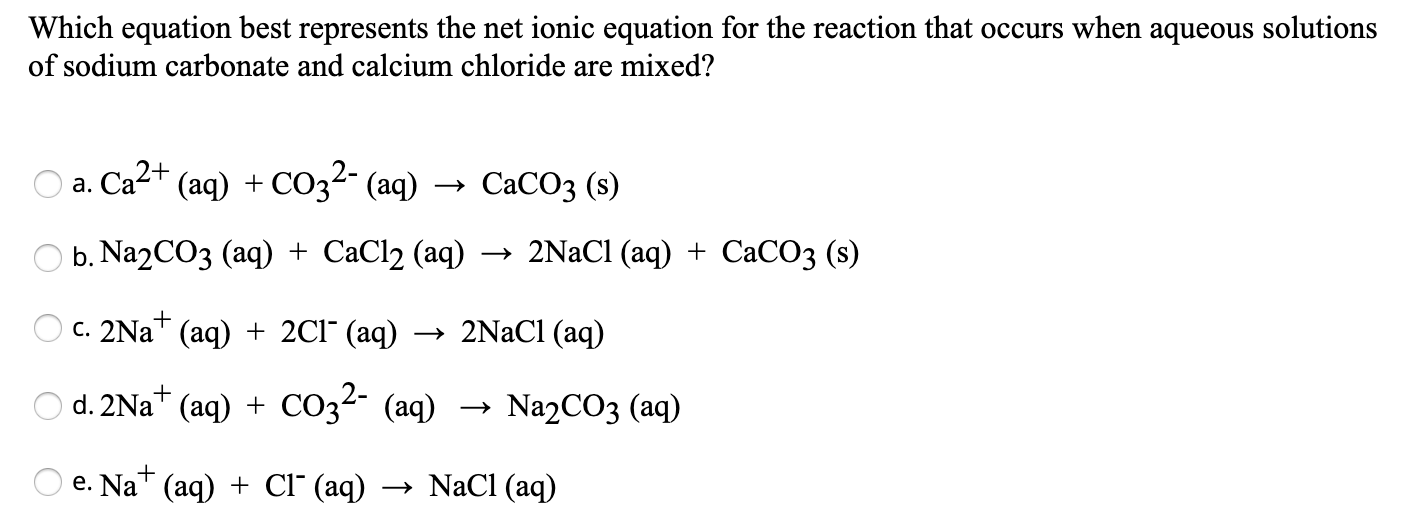Sodium Carbonate And Calcium Chloride Net Ionic Equation
Hey there, science explorers! Ever wonder what happens when you mix certain common chemicals? It’s like a tiny, invisible party happening in your beaker! Today, we’re going to peek at a particularly jazzy chemical get-together: the one involving sodium carbonate and calcium chloride. Now, that might sound a bit like a mouthful, but trust me, the outcome is pretty neat, and the way we describe it, called the net ionic equation, is where the real fun begins.
Imagine you have two different solutions, like two colorful drinks. In one is our star, sodium carbonate. Think of it as a bunch of happy little sodium ions (let's call them the Na+ crew) and carbonate ions (the CO32- gang) who are all dissolved and happily floating around. They’re just chilling in the water, not really bonded tightly, but spread out.
In the other beaker, we have calcium chloride. This one’s got its own crew of ions: calcium ions (the cool, laid-back Ca2+ dudes) and chloride ions (the energetic Cl- buddies). Again, they’re all dissolved and mingling freely in the water. It’s like a party where everyone’s invited and mingling.
Now, here’s where the magic happens. We pour these two solutions together. It’s like mixing those two colorful drinks. At first, it looks like everything is just going to keep on mingling. All four types of ions – Na+, CO32-, Ca2+, and Cl- – are still floating around. They’re all dissolved, so from a distance, you might not see much change.
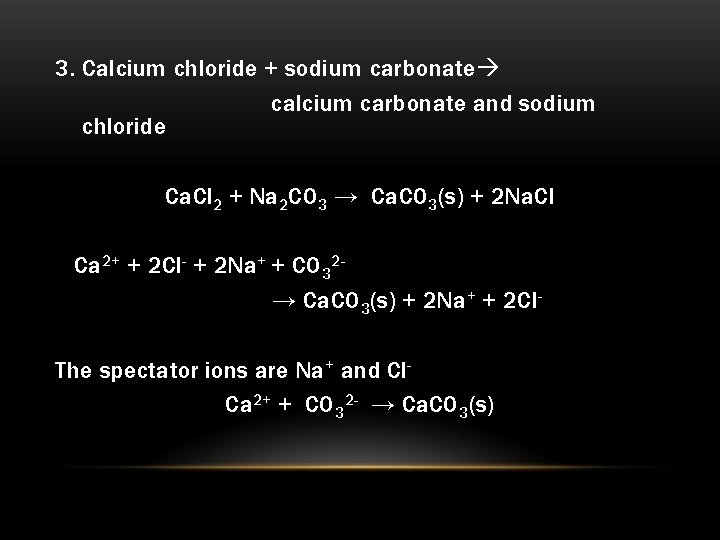
But wait! Some of these ions have a secret attraction. The Ca2+ dudes and the CO32- gang? They absolutely love each other. Like, really, really love each other. When they meet, they decide to ditch the water and form a brand new, super-strong bond. They decide to become calcium carbonate. And here’s the kicker: calcium carbonate is not very good at dissolving in water. It’s like they decided to form their own exclusive club, a solid club, a precipitate!

Suddenly, our clear liquid starts to get a little cloudy. That cloudiness is actually tiny little solid particles of calcium carbonate forming. It’s like a miniature snowstorm happening right before your eyes!
So, what happens to the other ions? The Na+ and the Cl- ions? Well, they’re still around. They tried to bond, maybe, but they weren’t as successful. The Na+ ions are still happy floating around, and the Cl- ions are also still happy doing their own thing. They didn't form any new solid or do anything particularly exciting. They’re like the guests at the party who just keep dancing and chatting, unaffected by the main event.
This is where the net ionic equation comes in, and it’s so cool because it cuts out all the boring stuff! Think of it like a highlight reel of the chemical reaction. It only shows us the stars of the show – the ions that actually did something significant.

In our sodium carbonate and calcium chloride mix, the Na+ ions and the Cl- ions are just spectators. They’re there, but they don’t actively participate in forming the new solid. They’re called "spectator ions." They're like the friends who show up to cheer on the main performers but don't actually get on stage themselves.
The real action, the chemical drama, is between the Ca2+ and the CO32-. They are the ones that change from being dissolved and floating around to forming a solid. They are the ones that are directly involved in creating the new substance, the calcium carbonate.
So, when we write the net ionic equation, we’re basically saying: "Okay, everyone else, you can take a break. We only care about the ions that are actively changing and forming something new." It’s like zooming in on the most exciting part of a movie, ignoring all the filler scenes.
For this particular reaction, the net ionic equation is pretty straightforward and elegant. It shows us that:
Ca2+ (the calcium ions) + CO32- (the carbonate ions) → CaCO3 (the solid calcium carbonate)
Solved Which equation best represents the net ionic equation | Chegg.com
Isn’t that neat? It perfectly captures the essence of what happened. It tells us that calcium ions and carbonate ions got together and made solid calcium carbonate. The sodium and chloride ions are completely left out because they didn’t do anything besides hang out.
This is what makes the net ionic equation so special and entertaining. It’s a concise, powerful way to understand the core chemical transformation. It strips away the unnecessary and focuses on the genuine chemical change. It’s like finding the simplest, most beautiful explanation for something complex.
So, the next time you hear about sodium carbonate and calcium chloride, or any other chemical reaction, remember that there’s a whole story happening at the ionic level. And the net ionic equation is the way we get to see the most captivating part of that story, the part where new things are actually made. It’s a little bit of chemistry magic, all explained with remarkable clarity!