Silver Nitrate And Ammonium Carbonate Net Ionic Equation
Hey there, fellow humans! Ever found yourself staring at a mysterious label on a bottle in a science lab, or maybe just wondering what happens when you mix a couple of common household-ish chemicals? Today, we're going to dive into something that sounds a bit fancy, but trust me, it's like a friendly chat about how things react. We're talking about Silver Nitrate and Ammonium Carbonate, and more specifically, their "net ionic equation." Don't let the big words scare you! Think of it as the real story of what’s actually happening at the tiniest level when these two meet.
Imagine you’re at a potluck dinner. Lots of people bring dishes. You’ve got your Aunt Carol’s famous seven-layer dip, your neighbor Dave’s surprisingly delicious Jell-O salad, and maybe even some questionable casserole from Uncle Bob. When you start dishing out your plate, you’re not really worried about which specific molecule of Aunt Carol’s sour cream is landing next to which specific molecule of Dave’s lime Jell-O, right? You’re just interested in what ends up on your plate after everyone has mingled.
Chemical reactions can be a bit like that potluck. When we mix Silver Nitrate and Ammonium Carbonate, we’ve got a whole bunch of different "ingredients" floating around. Silver Nitrate is like a party guest wearing a silver pendant (that's the silver ion, Ag+) and a nitrate party hat (the nitrate ion, NO3-). Ammonium Carbonate is a whole group of friends: some are carrying ammonium balloons (the ammonium ions, NH4+) and others have carbon dioxide party favors (the carbonate ions, CO32-). When you mix them, everyone starts mingling!
In the grand scheme of things, not everything that’s floating around in the liquid actually changes. Some of these ions are like those guests at the potluck who just stand around, chat with everyone, but don’t actually end up on your plate. They’re just there, observing the action. In chemistry terms, we call these "spectator ions". They’re spectators because they’re watching the main event, but they don't directly participate in the new thing that’s being formed.
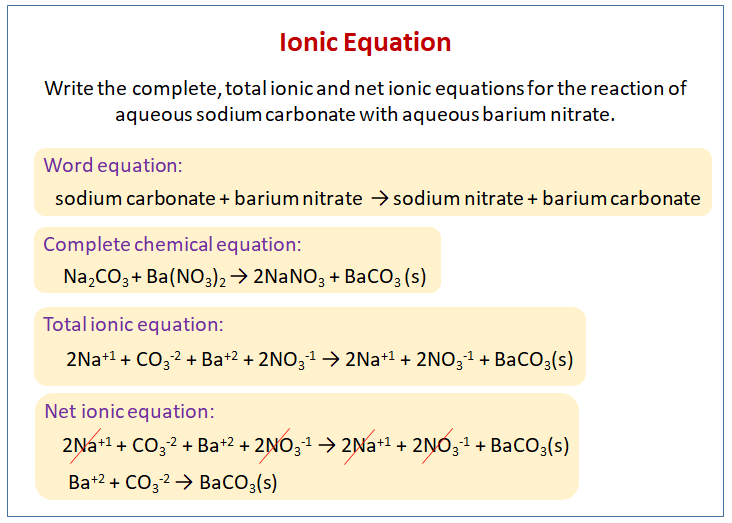
So, what’s the "main event" here? What’s the new dish being created at our chemical potluck? When Silver Nitrate and Ammonium Carbonate meet, a fascinating transformation occurs. The silver ions and the carbonate ions decide they’d rather team up. They form something new and solid, which we call silver carbonate (Ag2CO3). This is like when you and your best friend decide to ditch the big group and go grab a pizza just the two of you. You’ve formed a new, special pairing!

This silver carbonate is pretty unique because it doesn't dissolve in water. It sinks to the bottom, or floats around as a fine powder. In chemistry, we call this a precipitate. Think of it like that one guest who brings a giant, beautiful, and very solid fruitcake to the potluck. It doesn't just melt away; it’s a distinct thing you can see and touch.
Now, what about the other ions? The nitrate ions and the ammonium ions? They were all mixed up in the beginning, and guess what? They’re still mixed up and floating around in the liquid afterwards! They didn’t grab onto each other to form anything new that would sink or change. They’re the ones who went to the potluck, chatted with everyone, but didn’t end up on your plate as a distinct "dish." They are our spectator ions.
The net ionic equation is basically the simplified, "no-fluff" version of this reaction. It only shows the real players – the ones that actually changed and formed something new. It’s like getting the recipe for just the main dish, leaving out all the side details that didn't really contribute to its creation.
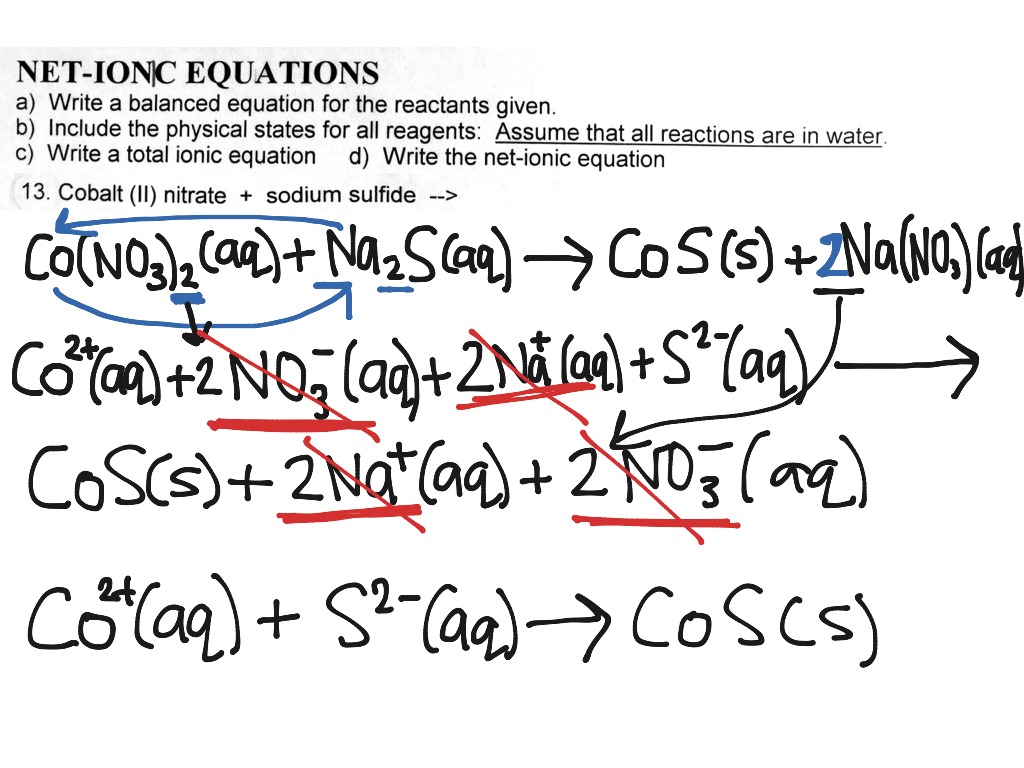
Let’s break it down a bit more. When Silver Nitrate (AgNO3) dissolves in water, it splits into its silver ions (Ag+) and nitrate ions (NO3-). When Ammonium Carbonate ((NH4)2CO3) dissolves in water, it splits into ammonium ions (NH4+) and carbonate ions (CO32-). So, initially, we have a watery soup of: Ag+, NO3-, NH4+, and CO32- all zipping around.
When these react, we know the silver (Ag+) and carbonate (CO32-) ions team up to form solid silver carbonate (Ag2CO3). This is the new thing! The nitrate (NO3-) and ammonium (NH4+) ions just keep doing their own thing, still dissolved in the water.
The full equation, showing everything, would look something like: 2AgNO3(aq) + (NH4)2CO3(aq) → Ag2CO3(s) + 2NH4NO3(aq) See all those "aq" (aqueous, meaning dissolved in water) and "s" (solid)? That tells us what’s what.
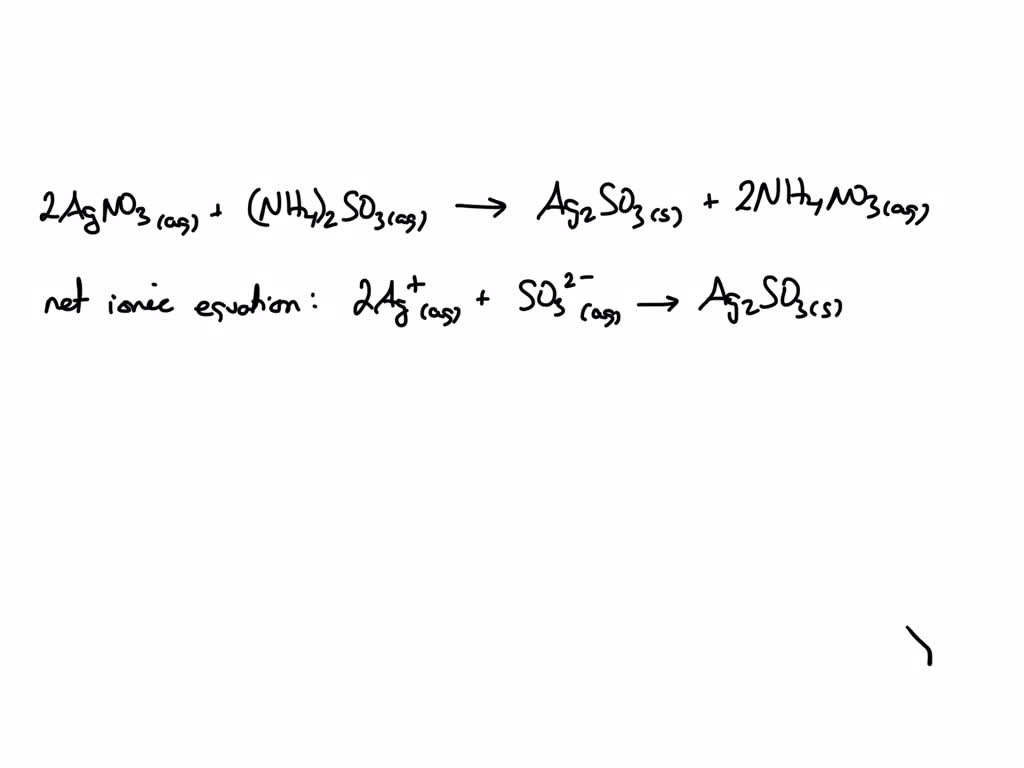
But the net ionic equation is much cleaner. It strips away the spectators. It says: 2Ag+(aq) + CO32-(aq) → Ag2CO3(s) This equation tells you the essential story: silver ions and carbonate ions get together to make solid silver carbonate. The ammonium and nitrate ions? They were there, but they didn't contribute to forming the precipitate. They are the folks who came to the party, saw the main attraction, but didn't become part of it.
Why should you care about this? Well, think about it! This little dance of ions is happening all around us, even if we don't see it. It's the basis for things like detecting certain substances, creating new materials, and even understanding how our bodies work at a microscopic level. When chemists want to create a specific medicine or a new type of material, they need to know exactly which parts of their ingredients are going to do the actual work and which are just along for the ride.
It's like knowing that to bake a cake, you need flour, sugar, eggs, and butter to form the cake. Water and baking soda are important too, but they participate in a slightly different way, creating the fizz and lightness. The net ionic equation is like focusing on the core ingredients that actually build the cake structure.
Understanding this "net" reaction helps scientists and engineers predict what will happen when they mix things. It's like knowing that if you mix baking soda and vinegar, you'll get bubbles (carbon dioxide gas). You don't need to worry about every single molecule of each, just the key players that create that exciting fizz!
So, next time you hear about a chemical reaction, whether it's in a lab, a cooking show, or even just a fascinating documentary, remember our potluck analogy. There are always the main actors who create the new, exciting substance, and there are the bystanders, the spectators, who just hang out. The net ionic equation is our way of highlighting the true stars of the show!
It’s a neat little way to simplify complex processes and get to the heart of what’s really going on. And honestly, who doesn't love a good simplification that reveals a hidden truth? It's like finding out your favorite song has a secret, simpler melody underneath all the instruments. Pretty cool, right?
