Show How 2-iodo-2-methylpropane Could Be Prepared From 2-methylpropane
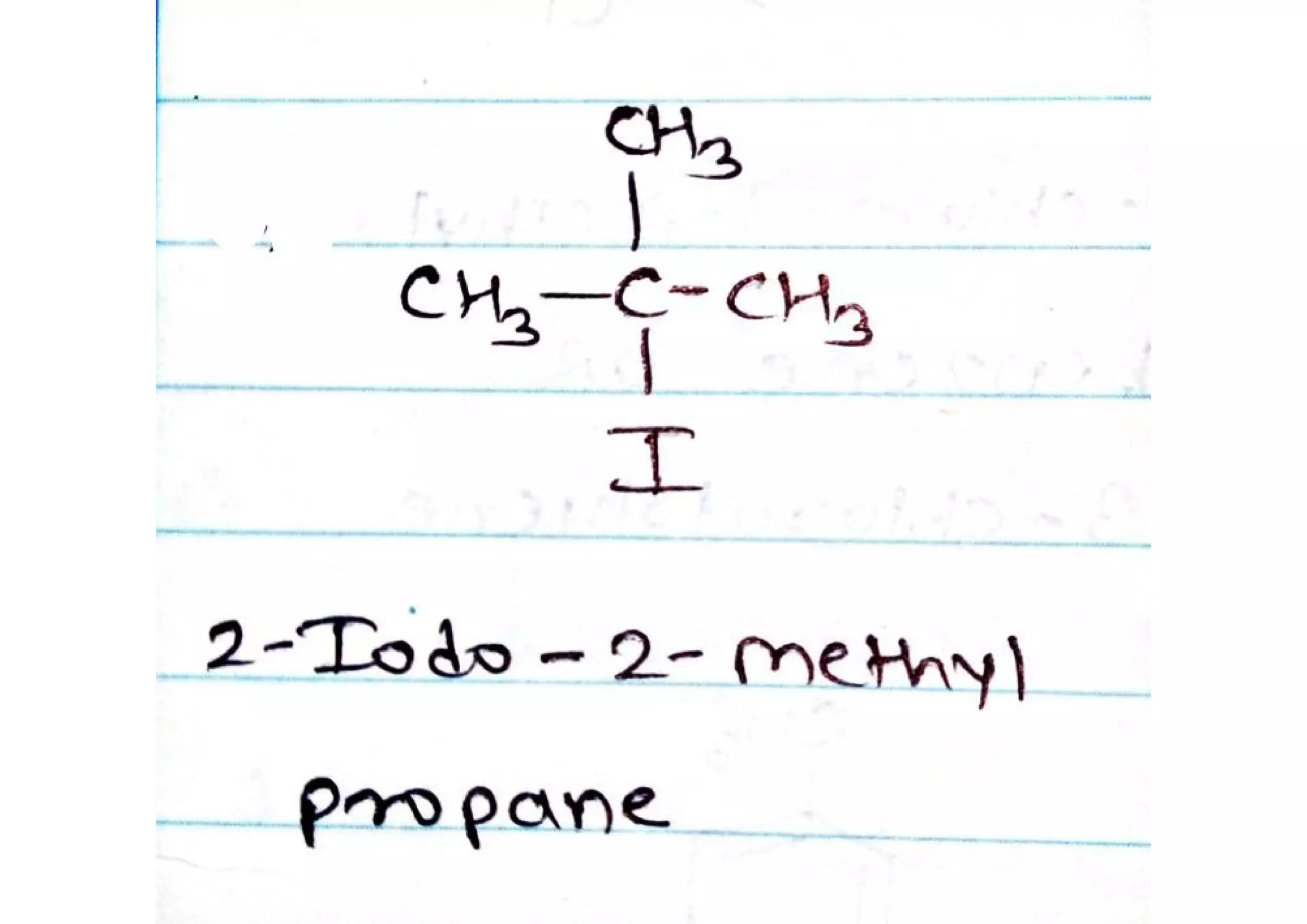
Hey everyone! So, have you ever looked at a simple molecule like 2-methylpropane – you know, that branched-out alkane stuff – and wondered, "Could I make something a bit more… exciting out of this?" Well, today we're going to dive into a surprisingly cool way to transform good ol' 2-methylpropane into something a little more snazzy: 2-iodo-2-methylpropane. Think of it like taking a plain vanilla ice cream and adding some fancy sprinkles and a cherry on top. It’s still fundamentally the same base, but with a whole new personality!
Now, before we get too deep into the chemical wizardry, let's get familiar with our starting material. 2-methylpropane, also known as isobutane, is pretty much the poster child for a simple, saturated hydrocarbon. It's like the comfy, worn-in jeans of the chemical world – reliable, straightforward, and not much to look at on its own. It’s made up of carbon atoms all linked together with single bonds, and each carbon is holding onto as many hydrogen atoms as it possibly can. Hence, "saturated." It's already pretty happy as it is, wouldn't you agree?
But where's the fun in staying comfortable all the time, right? Sometimes, we want to add a bit of oomph. And in the world of chemistry, that "oomph" often comes in the form of different functional groups. Our goal today is to swap out one of those hydrogens for an iodine atom. Why iodine, you ask? Well, iodine is a pretty neat element. It’s heavy, it’s a bit of a loner in chemical reactions, and when it attaches to a carbon, it can really change how that molecule behaves. It’s like adding a special key that can unlock a whole new set of doors for further reactions. Pretty neat, huh?
So, how do we achieve this transformation from the humble 2-methylpropane to the slightly more sophisticated 2-iodo-2-methylpropane? It’s not as simple as just, you know, waving a magic wand. We need a plan, a chemical recipe. And like any good recipe, we need the right ingredients and the right conditions.
The Not-So-Direct Route: Why a Direct Substitution Isn't Our First Choice
Now, your first thought might be: "Can't I just chuck some iodine at 2-methylpropane and call it a day?" If only it were that easy! Alkanes like 2-methylpropane are notoriously unreactive. They're like a well-fortified castle – everything is tightly bound and not easily dislodged. Direct substitution of a hydrogen with iodine, especially under mild conditions, just doesn't happen readily.
Think about it like trying to pry a LEGO brick out of a perfectly constructed LEGO castle. It's possible, but it'll take a lot of force and might damage the surrounding structure. We want a more elegant solution, one that’s less like brute force and more like a carefully planned heist.
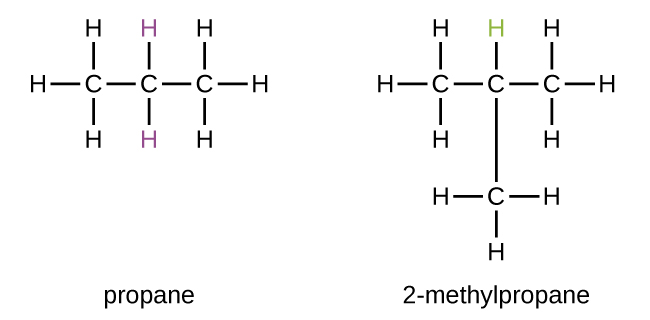
The challenge with directly substituting a hydrogen in an alkane is that all the hydrogens are pretty much the same. For 2-methylpropane, we have primary hydrogens (on the end carbons) and a tertiary hydrogen (on the central carbon). If we were to force a reaction, we’d likely get a messy mixture of products, and that’s usually not what we want. We want control, precision, and a clear path to our desired molecule.
Introducing Our Key Player: A "Halogenation" Reaction, But with a Twist!
Okay, so direct substitution with iodine is out. What’s our next move? We need to make our 2-methylpropane more susceptible to reaction. And one of the most common ways to do this is through something called halogenation. But we’re not going to be directly adding iodine just yet. Instead, we’re going to use a clever intermediate step.
The real magic happens when we first convert our 2-methylpropane into something that can react more easily. And for this, we often turn to other halogens, like bromine or chlorine, because they're a bit more cooperative in these types of reactions. But don't worry, we're still aiming for that iodine at the end of the day!
Let's consider a common and effective pathway. We'll start by reacting 2-methylpropane with a source of bromine, usually in the presence of light or heat. This is a type of free radical halogenation. Think of it like setting up a small, controlled spark that starts a chain reaction. This process will preferentially attack the tertiary carbon atom in 2-methylpropane. Why the tertiary carbon? Because the radical formed at the tertiary position is the most stable, like a well-balanced gymnast holding a tricky pose – it’s got good stability.

This reaction would give us 2-bromo-2-methylpropane. So, we’ve swapped a hydrogen for a bromine. We're one step closer! It’s like we’ve upgraded our plain vanilla to a chocolate swirl. We’ve introduced a reactive handle.
From Bromine to Iodine: The Halogen Exchange
Now we have 2-bromo-2-methylpropane. This molecule is much more amenable to further manipulation. We have our bromine atom sitting on the tertiary carbon, like a flag planted on a strategic hill. What we want, remember, is an iodine. So, how do we swap that bromine for an iodine? This is where a really cool reaction called a Finkelstein reaction comes into play.
The Finkelstein reaction is a classic way to perform a halogen exchange. It’s like trading one type of coin for another, where one is considered more valuable or useful for your purposes. In this case, we're trading a bromine atom for an iodine atom.
The typical setup for a Finkelstein reaction involves using an alkali metal iodide, most commonly sodium iodide (NaI) or potassium iodide (KI), dissolved in a suitable solvent, often acetone. Acetone is a great solvent for this because it readily dissolves the sodium or potassium iodide, and importantly, the sodium bromide or potassium bromide that’s formed as a byproduct is not very soluble in acetone. This insolubility is key!

So, what happens? When we mix our 2-bromo-2-methylpropane with sodium iodide in acetone, the iodide ion (I⁻) acts as a nucleophile. It’s like a little scout looking for a place to land. It attacks the carbon atom that’s bonded to the bromine. The bromine atom, being a good leaving group, detaches itself as a bromide ion (Br⁻).
This is an SN2 reaction mechanism at play, if you're into that kind of detail. The nucleophile (iodide) attacks the carbon from the backside, pushing the leaving group (bromide) off. It's a bit like a perfectly timed pass in a sports game, where the receiver catches the ball just as the defender is moving away.
Because sodium bromide (NaBr) is largely insoluble in acetone, it precipitates out of the solution. This is a super clever trick of chemistry! According to Le Chatelier's principle, when a product is removed from a reaction mixture, the equilibrium shifts towards the formation of more products. So, the removal of NaBr drives the reaction forward, ensuring that we get a good yield of our desired 2-iodo-2-methylpropane.
It’s a beautiful example of how understanding solubility and reaction equilibria can lead to efficient synthesis. We've taken a molecule that was a bit tricky to directly functionalize, converted it into a more reactive intermediate, and then performed a neat little swap to get exactly what we wanted. From a simple alkane to a tertiary alkyl halide, all with a couple of well-chosen steps!
Why is This Cool?
So, why is this whole process neat? Well, for starters, it shows that even seemingly simple molecules can be transformed into more complex or useful ones with a bit of chemical know-how. It’s like unlocking hidden potential. 2-methylpropane is abundant and cheap, and being able to modify it into a more reactive compound opens doors to all sorts of other chemical reactions.
Think of 2-iodo-2-methylpropane as a versatile building block. The iodine atom is a great leaving group, meaning it can be easily replaced by other atoms or groups of atoms. This makes it a valuable starting point for synthesizing pharmaceuticals, agrochemicals, or other specialty organic compounds. It’s the difference between having raw ingredients and having a pre-made component that can be easily incorporated into a larger structure.
Moreover, this process highlights the power of multi-step synthesis. Often, the most straightforward way to make a molecule isn't the best or most efficient. By breaking down a complex transformation into smaller, manageable steps, chemists can achieve elegant and high-yielding results. It’s like solving a puzzle by assembling smaller, interconnected pieces.
So, the next time you see something as simple as 2-methylpropane, remember that with a little ingenuity and the right chemical reactions, you can turn it into something much more interesting and useful. It's a testament to the creative and problem-solving nature of chemistry. Pretty awesome, right?
