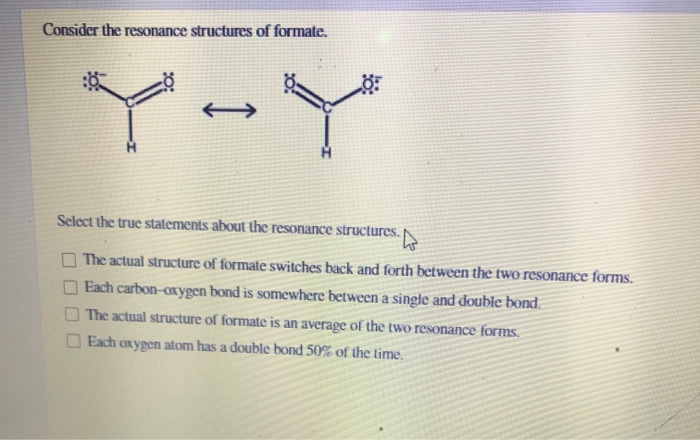
Select The True Statements About The Resonance Structures
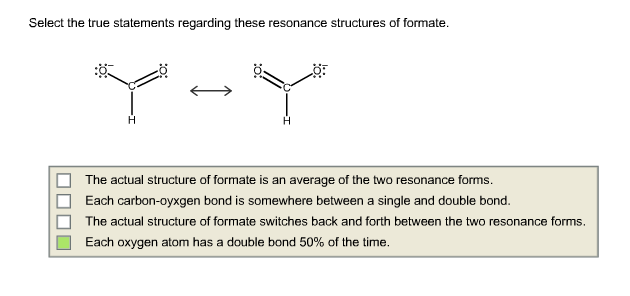
Ever wondered about the secret lives of atoms and molecules? It turns out they have a super fun, almost magical way of presenting themselves! We're talking about something called resonance structures. It's like a molecular fashion show where the same outfit can be styled in a few different, equally fabulous ways.
Imagine you have a favorite toy. Sometimes, you might play with it in one way, and then later, you might hold it a little differently, or attach a new accessory. The toy itself doesn't change, but how you see it or interact with it can. That's a bit like what happens with resonance structures.
In the world of chemistry, molecules are constantly showing off their possibilities. They aren't just stuck being one thing. Instead, they can have multiple "versions" of themselves that are all equally valid. These versions are what we call resonance structures.
Think of it like a chameleon. A chameleon can be green, or it can be brown, or even speckled. But it's still the same chameleon underneath all those colors. Similarly, a molecule that exhibits resonance isn't actually flipping between these structures. It's like it's a blend of all of them at once.
This "blend" is super important because it tells us a lot about the molecule's actual behavior. It's not just about pretty pictures; it's about understanding how things work at a tiny, tiny level. And that, my friends, is pretty darn cool.
So, what are the true statements about these dazzling resonance structures? It's like a game of "spot the real deal." We're looking for the descriptions that capture the essence of this molecular versatility.
First off, one of the most exciting things is that resonance structures are not real, individual molecules. This sounds a bit mind-bending, right? It's like saying your reflection in the mirror isn't you, but a representation of you. The actual molecule exists as a sort of average, a hybrid of all the possible resonance structures.
This hybrid form is what we call the resonance hybrid. It's the true, stable form of the molecule. The resonance structures themselves are just tools, like snapshots, to help us visualize and understand this hybrid. They're like the different poses a dancer strikes; the dance itself is the continuous motion, not just a single pose.
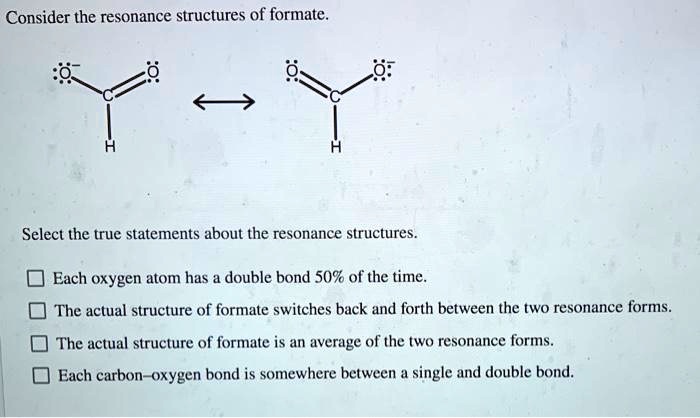
Another key point is that all resonance structures for a given molecule contribute to the resonance hybrid. It's not like one structure is the "best" and the others are just okay. Nope, they all play their part in painting the complete picture. Imagine a committee deciding on a design; each member's input is considered.
Some resonance structures might be more "influential" than others, meaning they contribute more to the hybrid's character. Think of it as some committee members having louder voices or more experience. These more influential structures are usually the ones that are more stable themselves.
So, how do we know which structures are more stable? Well, there are some handy rules of thumb. For instance, structures where atoms have a complete octet of electrons are generally more stable. It's like everyone at a party having their share of snacks; everyone's happier and more content.
Another stability booster is having more bonds. More connections often mean more stability. It's like a well-built structure with lots of support beams; it's less likely to topple over.
And, importantly, structures where negative charges are on more electronegative atoms and positive charges are on less electronegative atoms are also more stable. Imagine a grumpy cat being in its own cozy bed (electronegative atom) rather than in a place it dislikes. It's just happier there!
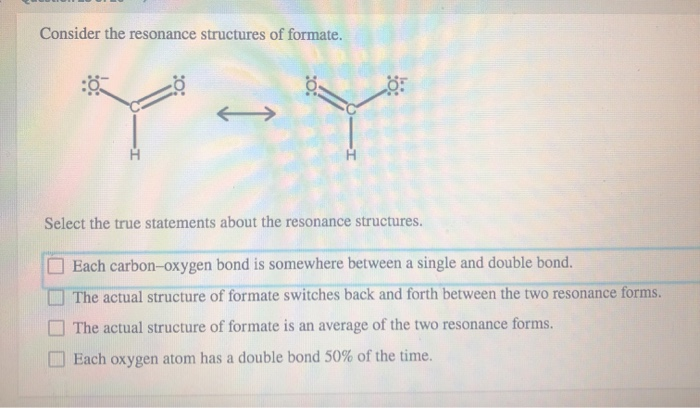
Also, a really crucial aspect is that atoms themselves do not move between resonance structures. What moves are the electrons, specifically the delocalized electrons. Think of it like a game of musical chairs, but instead of people moving, it's just the chairs rearranging. The people (atoms) stay put.
These moving electrons are often found in double or triple bonds, or in lone pairs. They are like free spirits, able to wander and share themselves around the molecule. This "sharing" is what creates the different resonance forms.
So, when we draw resonance structures, we use a special arrow. It's not a regular arrow that shows a reaction happening. Instead, we use a double-headed arrow. This arrow signifies that the structures are interconverting or are in resonance with each other. It’s like a handshake between different possibilities.
This double-headed arrow is super important! If you see a single arrow, that usually means a reaction is occurring, where one thing turns into another. But with resonance, it's more like they are siblings, all part of the same family, just looking a bit different in the photos.
It's also true that resonance helps to explain the observed properties of a molecule. For example, it can tell us why a bond might be shorter or longer than expected, or why a molecule is particularly reactive or stable. It's like understanding a person's personality helps you predict how they might act in certain situations.
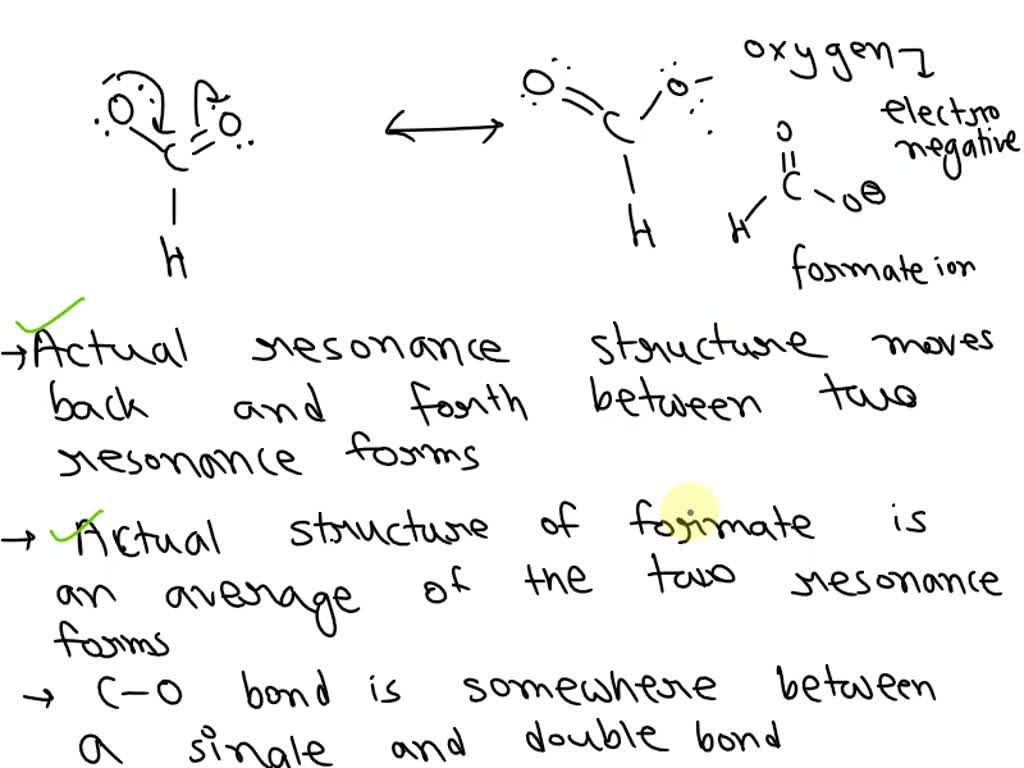
Take benzene, for example. It's a classic case. Benzene has a ring of six carbon atoms. If it were just a simple molecule with alternating single and double bonds, we'd expect two different types of carbon-carbon bonds: one long (single) and one short (double). But that's not what we see!
In benzene, all the carbon-carbon bonds are the same length, somewhere in between a single and a double bond. This is a direct consequence of resonance! The electrons are delocalized all around the ring, creating a hybrid structure where all the bonds are equal. It's like a perfectly balanced seesaw.
So, when you're looking at resonance structures, remember:
- They are not real, individual molecules.
- They are contributors to a resonance hybrid.
- Atoms stay put; only electrons move.
- A double-headed arrow connects them.
- They help explain a molecule's behavior.
It's this ability to represent molecules in multiple ways, and to understand that the "true" molecule is a blend, that makes resonance so fascinating. It challenges our simple, rigid ideas of what a molecule "is" and opens our minds to a more fluid, dynamic reality.
It's like having a collection of incredibly detailed sketches of a sculpture. Each sketch shows a different angle, a different play of light. But the sculpture itself is the solid, three-dimensional object that encompasses all those views.
The beauty of resonance structures is in their representation of electron delocalization. This sharing of electrons across multiple atoms makes molecules more stable and influences their reactivity in predictable ways. It's a fundamental concept in chemistry that unlocks a deeper understanding of the molecular world.
So next time you encounter resonance structures, don't just see them as complicated drawings. See them as glimpses into the dynamic, interconnected nature of molecules. They are the molecular equivalent of a perfectly blended smoothie, where all the ingredients work together to create something delicious and unique. It’s a little bit of molecular magic that makes chemistry so incredibly engaging!
Learning about resonance structures is like discovering a hidden feature in your favorite video game. It adds a whole new layer of complexity and coolness that you wouldn't have guessed was there. It's truly captivating!
The concept of resonance structures is a testament to the fact that the world at the atomic level is far more intricate and beautiful than we might initially imagine. It’s a constant reminder that there’s always more to explore and understand.
It’s this flexibility and interplay of electrons that makes molecules behave the way they do. It's why some reactions happen easily and others are more challenging. Understanding resonance is like getting a secret decoder ring for the language of chemistry.
So, delve into the world of resonance structures. Explore the different possibilities. See how these "imaginary" drawings unlock the real secrets of molecules. You might just find it more entertaining and insightful than you ever expected! It’s a journey into the heart of matter, and it’s full of delightful surprises.
