Select The High-energy Form Of Adenosine From The Following Images.

So, I was staring at my computer screen the other day, feeling that familiar midday slump. You know the one. It’s like your brain decides it’s time for a nap, and your fingers just… stop cooperating. I was contemplating a particularly dense scientific paper, probably about some obscure biochemical pathway that felt a million miles away from my urgent need for a mental refresh. And then, out of nowhere, I remembered this totally wild dream I had a few nights prior.
In this dream, I was a tiny, microscopic explorer, navigating a bustling cityscape made of cells. The most fascinating thing was the energy currency everyone was using. It wasn't like cash, or even Bitcoin. It was these glowing, shimmering little packets that zoomed around, fueling everything from the cell’s construction projects to its frantic communication systems. It was so vivid, I swear I could almost feel the hum of all that activity.
Fast forward to my current predicament: that dense scientific paper. It was all about adenosine, a molecule that, let’s be honest, sounds a bit like a fancy new sports drink. But it’s actually way more fundamental than that. It’s a building block, a messenger, and apparently, a key player in how our bodies get and use energy. And suddenly, my dream about those glowing energy packets clicked. I had a hunch that adenosine, in its most energetic form, was exactly what I was seeing in my dream. Pretty cool, right? It made me think, if you’re going to be talking about energy, you gotta talk about the high-octane version of the molecules involved.
So, the mission, should you choose to accept it (and hey, you’re already here, so I’m guessing you are!), is to figure out which of the following images shows the high-energy form of adenosine. It’s like a microscopic scavenger hunt, but way more important for understanding how you, me, and pretty much everything alive on this planet, keeps going. Think of it as upgrading your biological understanding from dial-up to fiber optic, just by looking at a few pictures. You ready to power up your knowledge?
The Humble Beginnings: Adenosine Itself
Before we dive into the electrifying stuff, let’s get acquainted with the basics. Adenosine, at its core, is a molecule made up of two parts: an adenine base (which is one of the letters in our DNA, remember A, T, C, G? That’s the ‘A’!) and a ribose sugar. Think of it like a basic LEGO brick. It’s useful, it’s fundamental, but it's not exactly doing backflips of excitement on its own.
This base adenosine molecule is crucial, no doubt. It's involved in all sorts of cellular processes. But when we talk about energy, we’re usually talking about something a little… more. It’s like comparing a car that’s parked to a car that’s just put the pedal to the metal. Both are cars, but only one is going places fast.
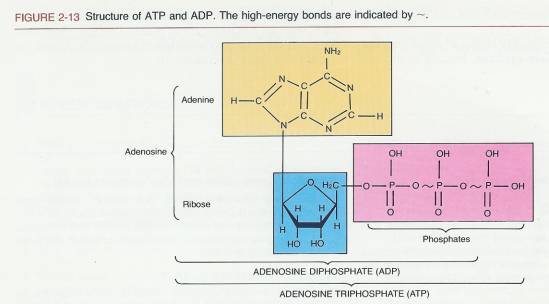
So, while adenosine is the foundation, the star of our energy show is going to be its souped-up, ready-to-rumble cousins. You’ve probably heard of ATP before, right? That’s Adenosine Triphosphate. The ‘tri’ means three, and in this case, it refers to three phosphate groups attached to our humble adenosine. And that, my friends, is where the magic happens. Or, more accurately, where the energy is stored.
Phosphates: The Energy Superchargers
Let’s get a little nerdy for a second, but I promise to keep it fun. Imagine those phosphate groups as tiny, tightly coiled springs. When you add them to adenosine, one by one, you’re essentially coiling those springs tighter and tighter. Each phosphate group that gets attached requires a significant amount of energy to bond.

So, adenosine monophosphate (AMP) has one phosphate. Adenosine diphosphate (ADP) has two. And then we get to Adenosine Triphosphate (ATP) with a whopping three phosphate groups. Think of AMP as a slightly wound spring, ADP as a moderately wound spring, and ATP as that spring that’s about to snap if you coil it any tighter. This stored energy is released when those high-energy phosphate bonds are broken. It’s the biological equivalent of a tightly wound rubber band snapping!
The breaking of that bond between the second and third phosphate group in ATP is what releases a burst of energy that our cells can use. It’s the universal energy currency. When your muscles contract, when your brain thinks, when your body repairs itself – chances are, ATP is involved. It’s literally powering you from the inside out.
So, when we’re looking for the high-energy form of adenosine, we’re not just looking for adenosine floating around. We’re looking for adenosine that has hitched a ride with a bunch of these energy-packed phosphate groups. It’s like looking for the most exciting person at a party – they’re usually the ones with the most dynamic accessories!
Deciphering the Images: A Visual Quest
Now, let’s get to the fun part – the pictures! Your task, should you choose to accept it, is to cast your discerning eye upon the provided images and identify the molecule that screams "ENERGY!" It’s not always obvious at first glance, is it? Science can be a bit like a secret code sometimes, and these molecular diagrams are part of that code.
When you look at these images, what should you be looking for? You’re looking for that adenosine structure (the adenine and the ribose sugar) and then, crucially, the phosphate groups attached to it. The more phosphate groups, and the way they are linked, the more likely it is to be the high-energy form.

Think of it this way: if you see a simple adenosine molecule, that’s like seeing a plain battery. Useful, but not exactly generating a lightning bolt. If you see adenosine with one phosphate group (AMP), that’s like a battery with a small charge. Better, but still not quite there. If you see adenosine with two phosphate groups (ADP), you’re getting warmer. That’s a decent charge.
But the real showstopper, the one that’s going to be buzzing with activity, is the one with three phosphate groups. That’s ATP, the undisputed king of cellular energy. It’s the one that’s been coiled up tight, ready to unleash its stored power. So, keep an eye out for that distinctive chain of phosphate groups attached to the adenosine core. It's like spotting the superhero with their cape on and ready for action!
Image Analysis Checklist (Your Secret Decoder Ring!)
Let’s make this super clear. When you’re scrutinizing those images, here’s what’s going to guide you:
- Look for the Adenosine Backbone: This consists of the adenine ring and the ribose sugar. They’ll look like a specific arrangement of carbon, nitrogen, and oxygen atoms. It's the consistent part, the anchor for our energy party.
- Count the Phosphate Groups: This is the KEY. You're looking for structures that are essentially chains of phosphorus and oxygen atoms. The more phosphates in a row attached to the sugar, the better!
- Identify the High-Energy Bonds: In the most energetic forms, these phosphate-phosphate bonds are often depicted with a wavy line (~) in some diagrams. This wavy line is a special notation signifying a high-energy bond – the kind that, when broken, releases a substantial amount of energy. If you see those wavy lines connecting the phosphate groups, you’re practically staring at pure, unadulterated cellular power.
- Compare and Contrast: Don’t just pick the first thing that looks complicated. Compare the images side-by-side. One might be adenosine alone, another AMP, another ADP, and hopefully, one will be that glorious ATP. You’re looking for the one that has the most of those energy-packing phosphate groups.
It's like a game of "spot the difference," but the difference here is the amount of raw, usable energy packed into a single molecule. And trust me, when it comes to energy, more phosphate groups generally equals more bang for your biological buck.
The Reign of ATP: The Universal Energy Currency
So, by now, you’ve probably zeroed in on the winner. It’s the one with the adenosine and the triple threat of phosphate groups: Adenosine Triphosphate (ATP). This is the molecule that fuels everything from the microscopic dance of bacteria to the complex thoughts racing through your brain right now. It’s the ultimate energy voucher.
When a cell needs energy to do something – build a protein, pump ions across a membrane, or even just move – it breaks off one of those phosphate groups from ATP. This reaction releases energy, and what’s left is ADP (Adenosine Diphosphate) and a free phosphate. Think of it like spending a $100 bill to buy something; you get your item, and you’re left with change.
But here’s the clever part: our cells are constantly working to recharge that ADP back into ATP. They take energy from food (like glucose) and use it to stick that third phosphate group back onto ADP, reforming ATP, ready to be spent again. It’s a continuous cycle of energy capture, storage, and release. It’s brilliant, really. Our bodies are basically little energy recycling plants, and ATP is the main commodity.
The other forms, like AMP and ADP, are also important. They’re part of the ATP cycle. ADP is the "used" form, waiting to be recharged, and AMP is like the really, really depleted version. But when we talk about the high-energy form, we’re talking about the molecule that’s holding the most potential energy, the one that’s ready to power the next cellular event. And that, unequivocally, is ATP.
So, next time you feel a surge of energy, whether it’s from a good night’s sleep, a healthy meal, or just the sheer joy of understanding something new, you can thank ATP. It’s working its magic behind the scenes, ensuring that every single process in your body has the fuel it needs to keep you functioning. It’s the unsung hero of your biology.
Beyond the Images: Why This Matters
Why should you care about identifying the high-energy form of adenosine? Well, beyond just satisfying your curiosity (which is a perfectly good reason!), understanding ATP and its role is fundamental to understanding life itself. It’s the engine that drives cellular metabolism. Every single organism on this planet, from the tiniest bacterium to the largest whale, relies on this molecule for energy.
.jpg)
Think about diseases. Many diseases, from cancer to heart disease, involve disruptions in cellular energy production and utilization. If cells aren’t making enough ATP, or if they’re using it inefficiently, things start to go wrong. So, understanding ATP is key to developing new treatments and therapies.
Even something as simple as exercise is all about ATP. When you work out, your muscles are furiously using ATP to contract. Your body then ramps up its production of ATP to keep up with the demand. That’s why you get tired – your ATP stores can get depleted faster than your body can replenish them.
And then there’s the fascinating world of biochemistry and molecular biology. These fields are constantly exploring new ways to harness and manipulate energy at the molecular level. Understanding ATP is like having the Rosetta Stone for all of that research. It’s the fundamental language of cellular energy transfer.
So, the next time you see a molecular diagram, or hear about a new scientific discovery, remember that behind it all, there’s often a story about energy. And the high-energy form of adenosine, ATP, is at the heart of it. It’s a small molecule with a monumental impact. It’s the spark that ignites life.
I hope this journey into the world of adenosine and its energetic alter-ego has been as illuminating for you as it was for me to stumble upon it (and dream about it!). Remember, science is everywhere, even in the microscopic packets of energy that keep you going. Keep exploring, keep questioning, and keep powering up your understanding!
