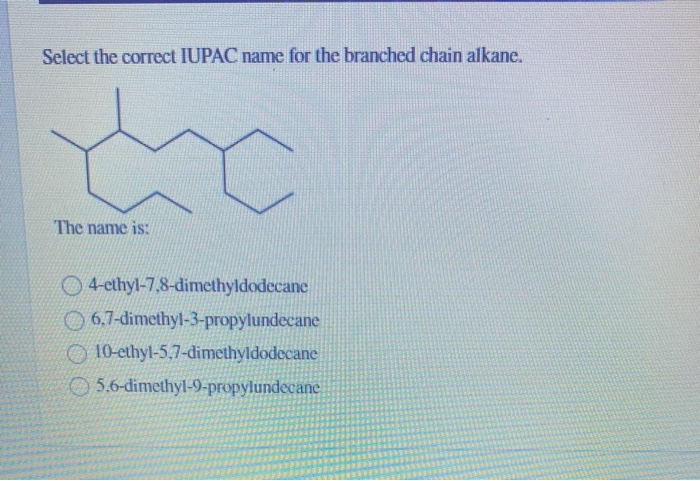
Select The Correct Iupac Name For The Branched Chain Alkane
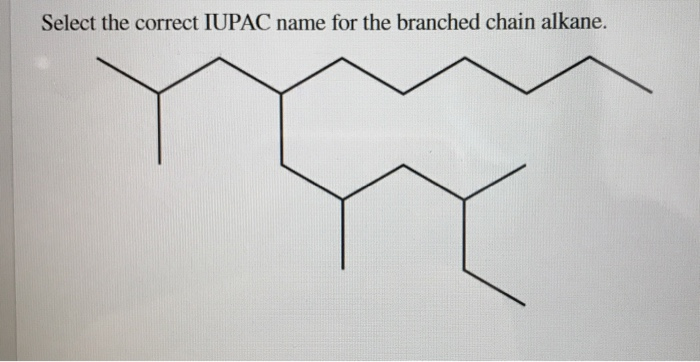
Hey there, science curious folks! Ever feel like you're looking at a jumble of letters and numbers, especially when it comes to, well, stuff? Like, what's the deal with all those complicated names for simple things? Today, we're diving into the wonderfully organized world of naming branched-chain alkanes. Think of it as giving every single house on a super-duper-messed-up street a proper address, so no one gets lost!
Now, I know what you might be thinking. "Alkanes? IUPAC names? Sounds like something from a chemistry textbook I tried to avoid in school." But stick with me! This isn't about memorizing a bunch of rules. It's about understanding a clever system that helps scientists (and even you!) talk about the building blocks of so many things we use every day. From the gasoline in your car to the plastic in your phone, alkanes are everywhere!
Why Should You Even Care About a Fancy Name?
Honestly, it’s all about clarity and communication. Imagine you're trying to order a very specific pizza. You wouldn't just say "pizza," right? You'd say "a large pepperoni pizza with extra cheese, no mushrooms." The more details you give, the more likely you are to get exactly what you want. IUPAC names are like those detailed pizza orders for molecules. They tell us exactly what a specific molecule looks like, down to the last atom.
Why is that important? Well, in science, precision is key. If two scientists are talking about the same chemical, they need to be 100% sure they're on the same page. Otherwise, you might end up trying to build a car with the wrong kind of fuel, and that, my friends, would be a bit of a disaster!
Plus, understanding how these names are put together is like learning a secret code. Once you crack it, you can start to understand the structure and properties of the molecules. It’s like knowing that a "ranch style" house probably has certain features, even if you haven't seen that specific house before. You can make educated guesses!
Let's Meet the Star of the Show: The Alkane
So, what exactly is an alkane? Think of them as the simplest kind of hydrocarbons. Hydrocarbons are just molecules made of hydrogen (H) and carbon (C) atoms. Alkanes are the "straight-laced" versions of these guys – meaning the carbon atoms are linked in a single, continuous chain. Like a perfectly straight line of dominoes.
We're talking about things like methane (one carbon, four hydrogens – CH4, the simplest!), ethane, propane, butane – the stuff that might be in your camping stove or even used for heating your home. These are the foundation. But what happens when things get a little more… interesting?
Enter the "Branched-Chain" Party Crashers!
This is where it gets fun! Imagine your straight line of dominoes. Now, imagine a few of those dominoes decide to go off on their own little adventures, attaching themselves to the main chain at different spots. That's a branched-chain alkane! It's still made of carbon and hydrogen, but the carbon skeleton isn't a simple straight line anymore. It’s got little side trips.
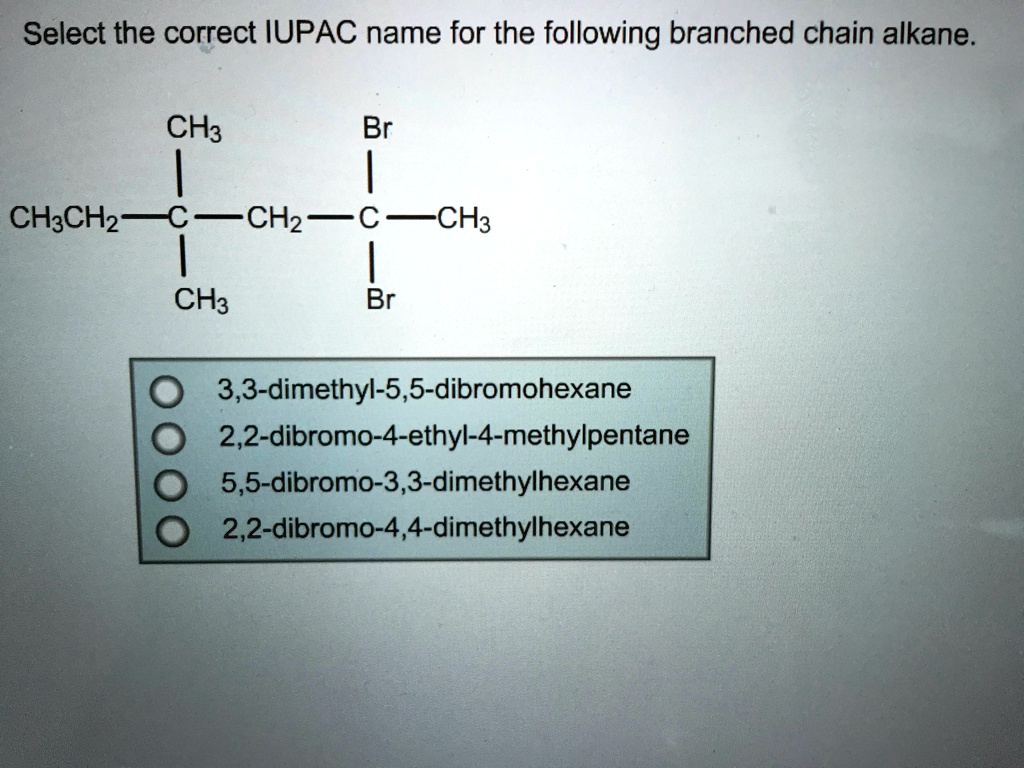
Think of it like a family tree. You have your main lineage, but then there are aunts, uncles, and cousins branching off. Or think of a highway system. You have the main interstate, but then there are all these smaller roads and exits that take you to different neighborhoods. These branches are what make molecules unique and give them different characteristics.
The IUPAC Detective Agency: How to Name Them
The International Union of Pure and Applied Chemistry (IUPAC) – that's the official name-giver – has a system, and it's actually pretty logical. It's like a detective solving a mystery. They need to identify the main culprit, then the accomplices, and figure out where everyone fits in.
Here’s the detective manual, simplified:
Step 1: Find the Longest Chain (The "Parent" Molecule)
This is your main clue. You look at the molecule and find the longest continuous chain of carbon atoms. This longest chain determines the base name of the alkane. If the longest chain has 5 carbons, the parent name is pentane. If it has 6, it's hexane, and so on.
Think of it like finding the oldest, most established branch of your family tree. That's the foundation you build from.
Step 2: Identify the "Branchers" (The Side Chains)
Once you've found your longest chain, look at what's attached to it that isn't part of that main chain. These are your branches. These branches are usually made of just one or two carbon atoms. If a branch has one carbon, it's called a methyl group. If it has two carbons, it's called an ethyl group.
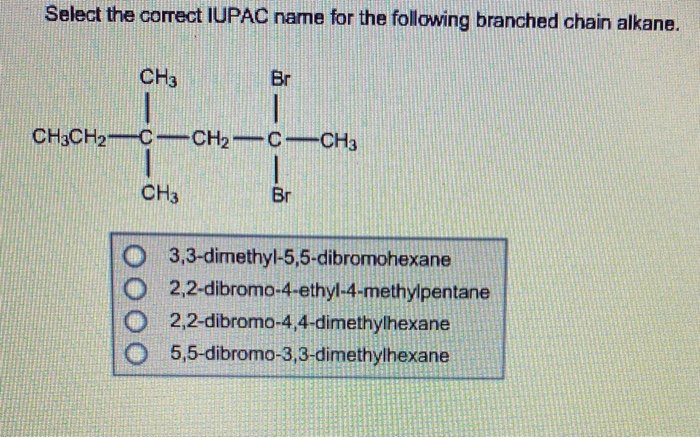
These are like the "extended family" members who have their own little houses but are still connected to the main neighborhood.
Step 3: Number the Parent Chain (The Address System)
Now, you need to give everyone an address. You number the carbon atoms in the longest chain. The trick here is to start numbering from the end that gives the branches the lowest possible numbers. It's like assigning house numbers – you want the street to make sense and be easy to navigate, starting from the most convenient end.
Imagine a street where houses are numbered 1, 2, 3, and then suddenly jumps to 10, 11, 12. That’s confusing! IUPAC wants the simplest numbering.
Step 4: Put It All Together (The Full Address)
Now you combine all the information. You state the location of the branch (the number on the parent chain), then the name of the branch, and finally the name of the parent chain. You use hyphens to separate numbers and letters, and commas to separate numbers.
Let's say you have a propane (3-carbon) chain. And on the second carbon atom of that chain, you have a methyl group (a single carbon branch). The IUPAC name would be 2-methylpropane.
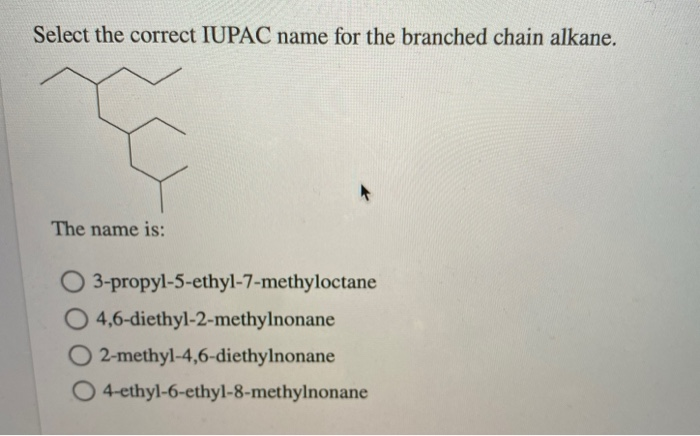
Sounds a bit like a real estate listing, right? "Charming 2-methylpropane with a cozy methyl extension on the second story."
Let's Try a Fun Example!
Imagine this molecule: A chain of 4 carbons. On the second carbon of that chain, there's a methyl group sticking out.
1. Longest chain: We have a 4-carbon chain. That makes our parent name butane.
2. Branches: We have one methyl group attached.
3. Numbering: If we number from the left, the methyl group is on carbon 2. If we number from the right, it's also on carbon 2. So, it doesn't matter which way we go!
4. Putting it together: The methyl group is on position 2, and the parent chain is butane. So, the name is 2-methylbutane.
See? It's like solving a little puzzle. And once you get it, you can look at other molecules and start to decipher their names too.
What if There's More Than One Branch?
If you have multiple branches, you list them all! If you have two methyl groups, you use the prefix di- (meaning two). So, dimethyl. If you have three, it's tri-, and so on.
And you list them in alphabetical order. So, if you have a methyl group and an ethyl group, the ethyl group comes first in the name, even if it appears later on the chain. It’s like organizing your bookshelf by author's last name, regardless of where the book is placed.
Why This Matters (Beyond Chemistry Class)
Understanding these names helps us appreciate the diversity of matter around us. It helps us understand how different arrangements of the same atoms can lead to completely different materials with different properties. It's like how the same Lego bricks can be used to build a car, a house, or a spaceship – the basic components are the same, but the arrangement makes all the difference.
So, the next time you see a complicated chemical name, don't run away! Think of it as a helpful address, a way for scientists to be super precise. And who knows, you might even find it a little bit fun to play "name that molecule"!
It’s all about making the world of chemistry a little less intimidating and a lot more understandable, one branched-chain alkane at a time. Happy naming!
